Bacterial Infections in the Poultry Sector
Amiya Ojha1*, Deeplina Das1 and Tarun Kanti Bandyopadhyay2
1Department of Bioengineering, NIT Agartala, Tripura-799046, India
2Department of Chemical Engineering, NIT Agartala, Tripura-799210, India
Introduction
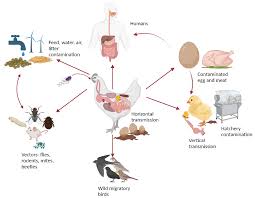
Bacterial infections and abnormalities are major concerns for the global chicken industry, resulting in huge economic losses and possible dangers to food security. Poultry, such as chickens, turkeys, ducks, and geese, are vulnerable to a wide range of bacterial infections that can cause a variety of diseases and health concerns (Elmberg, Berg et al. 2017). About half of the mortality in broiler breeders and commercial layers that is not connected to outbreaks is caused by bacterial infections. About half of the deaths of broiler chicks during their first week of life can be attributed to bacterial disease (Swelum, Elbestawy et al. 2021). Bacterial infection outbreaks can cause a sharp rise in mortality and, in certain situations, nearly wipe out flocks. Infections with Gram-positive cocci and E. coli cause mortality and production losses in chicken across all age groups and production methods; these infections may be complex in nature (Fancher, Zhang et al. 2020). These diseases not only affect the birds’ welfare but also impact the efficiency of production systems, leading to reduced productivity and increased mortality rates. The occurrence and severity of bacterial infections in poultry are determined by a number of factors, including management approaches, environmental conditions, and pathogen virulence (Deblais, Kathayat et al. 2020). Furthermore, the advent of antibiotic resistance in bacterial strains complicates disease control and treatment measures, demanding continued study and attention in the poultry industry.
In this paper, we will discuss at some of the most significant bacterial infections and disorders affecting poultry, concentrating on their causes, clinical symptoms, diagnostic techniques, preventive, and control strategies. By acquiring a better knowledge of these issues, we may strive to establish appropriate management techniques and treatments to reduce the impact of bacterial infections on chicken health and productivity. We hope to protect the health of chicken populations and preserve the poultry industry’s long-term viability in the face of bacterial threats through collaboration among academics, veterinarians, producers, and policy officials.
Bacterial Infections as a Cause of First-week Mortality (FWM)
Bacterial infections are the major cause of FWM. Kemmett et al. (2014) found that E. coli significantly contributes to chick mortality within 48-72 hours of implantation, despite a lack of relevant data (Kemmett, Williams et al. 2014). The study indicated that over 70% of the deceased chicks had colibacillosis, and the E. coli isolates had 30 distinct virulence profiles. Another investigation on 48-layer flocks found an average FWM of 1.4%. Over 50% of flocks experienced FWM owing to infectious causes, with yolk sac infection(YSI) and septicemia affecting 94% of them. Salmonella is the most commonly isolated genus from YSI in broiler and layer chicks, followed by Pseudomonas, Staphylococcus, Streptococcus, Proteus, Klebsiella, Entererococcus, Corynebacterium, Citrobacter, Aeromonas, Bacillus, Clostridium, Micrococcus, Yersinia, Enterobacter, Aerobacter, Achromobacter, and Alcaligenes leading to several economic losses. The most common organisms identified from these illnesses were E. coli and Enterococcus faecalis (Olsen, Frantzen et al. 2012). 2010-2011 research in Ethiopia found a 33.1% frequency of YSI in the FWM, mostly among 3- to 5-day-old chicks. E. coli was the most common bacterium found in these instances, followed by Staphylococcus aureus (Amare, Amin et al. 2013). . However, Karunarathna et al. (2017) reported the incidence of Enterococcus (29.71%) and E. coli (19.46%) isolated from dead chick embryos during the incubation (Karunarathna, Popowich et al. 2017).
Bacterial Contamination of Poultry Feeds
As a result, they cause human secondary infections and zoonotic disease epidemics that lead to significant hospitalization instances. Consequently, a poultry operation’s feed mill’s ingredient quality control section is a crucial initial step in keeping birds from contamination on the farm. suggested that increasing fecal contamination of feed supplies was a major cause of foodborne and waterborne diseases linked to Salmonella sp. (Crump, Griffin et al. 2002). The survey also reaffirmed that some farmers frequently use animal excrement as fertilizer for crops or as raw materials to make animal feed. Salmonella serotypes routinely discovered in processed poultry are frequently different from those found in feed components (Shah, Paul et al. 2017). It’s possible that using these raw materials will contaminate the feed mill’s surroundings later on. Sixty-eight percent of production expenditures are devoted to poultry feed, which is mostly made up of combinations of maize and soybean meal along with other vitamins and minerals and usually two or three pharmaceuticals (Njuguna 2007). Animal dung combined with soil can infect standing crops through fertilizer or direct deposition. Pathogens in the environment can be transported and stored by houseflies and cockroaches that consume excrement. The majority of plant-based diets for small animals are difficult to clean or sanitize. Consequently, there will be a chance that both customers and animals might contract foodborne illnesses. A more economical risk management approach to reduce pathogen proliferation in animal feeds would probably be to strengthen biosecurity protocols for feed storage at the feed mill or on the farm (Dewey, Bottoms et al. 2014).
Bacterial Contamination in Water Resources
The composition of water changes according to location and climate. If surface water seeps into the well, particularly if the water source is exposed, pollution of the water may result. Numerous studies have shown a correlation between the prevalence of E. coli O157:H7 in chicken excrement and pollution of drinking water(Ferens, Hovde et al. 2011, Saxena, Kaushik et al. 2015). When water quality is low, the efficacy of immunizations and drugs given through the water may be compromised. Because water sources continue to gradually become more contaminated due to urban and rural activities, water is a good means of transmission for agents responsible for human and animal illnesses, particularly those in which fecal-oral transmission occurs. revealed the prevalence of Vibrio cholerae, Salmonella typhimurium, and pathogenic E. coli in South African surface and groundwater sources (Momba, Malakate et al. 2006). Customers are seriously at risk for major health problems if these pathogenic bacteria are present in sources of drinking water. Reducing the amount of this harmful bacteria in poultry drinking water may be accomplished by eliminating E. Coli O157:H7, which is a worthwhile objective (Ferens, Hovde et al. 2011). Microbial contamination is a primary source of fecal contamination of water bodies, which can result in waterborne infections and be harmful to human health. Microbial water pollution may be caused by concentrated animal feeding operations (CAFOs). Particularly during rainy seasons, human excrement from these industries may find its way into rivers, lakes, and streams. Some of these pathogens from poultry manure can survive weeks in soil (Leighton 2018). This means that heavy precipitation events could also cause water contamination by collecting and discharging the slurry (soil and water mixture) into the closest body of water. Because antibiotics were once used to keep animals healthy and prevent possible infections as well as, until recently, to encourage development, they are still present in excrement at low quantities.
Escherichia coli infection
- coli belongs to the Enterobacteriaceae family and is a species in the Escherichia genus. This gram-negative, non-acid-fast, uniformly stained, and does not produce spores.
They may develop in both aerobic and anaerobic conditions. They can ferment glucose and produce gas in nutritional medium at temperatures ranging from 18 to 44°C.
E. coli causes turbidity in both cultures and often forms 1 to 3 mm colonies on blood agar. The shape of colonies varies, with rough colonies being bigger and with irregular margins, and smooth colonies being elevated, damp, and with well-defined borders. E. coli is typically found in the intestine, mucosal surfaces, avian skin, and feathers. The chicken gut contains around 106 colony-forming units of E. coli per gram of excrement (Lee 2022). These strains consistently belong to both pathogenic and nonpathogenic categories. Only some strains, known as APEC, have unique virulence characteristics that contribute to illness transmission in birds (Hu, Afayibo et al. 2022). Most of these infections are extraintestinal, with respiratory and systemic infections accounting for the vast majority. APEC has been classified as ExPEC because to its comparable pathogenicity to E. coli strains that cause urinary tract infections, sepsis, and neonatal meningitis (Hu, Afayibo et al. 2022). According to reports, APEC egg transmission occurs often and results in significant FWM when fluoroquinolone-resistant E. coli from clinically normal breeders is vertically transferred (Swelum, Elbestawy et al. 2021).
Mycoplasma infection
Mycoplasma is a tiny bacterium with around 25 distinct kinds. They lack a cell wall and are solely protected by a plasma membrane. The most prevalent economically severe kinds are Mycoplasma gallisepticum (MG) and Mycoplasma synoviae (MS) (Chaidez‐Ibarra, Velazquez et al. 2022). MG is responsible for a subclinical upper respiratory disease that is usually almost asymptomatic. If the infection is accompanied by other respiratory diseases, such as AI, ND, IBV, E. coli infection, and infectious synovitis when it is systemic, symptoms may manifest and may be severe. Mycoplasma may weaken the immune system, leading to significant morbidity and mortality in hens with chronic respiratory disease (CRD) (Luo, Fan et al. 2023). This can also diminish bird performance and increase condemnation rates during sale. Avian mycobacteriosis is a chronic illness in chickens that causes increasing loss of condition. Even with regular feed consumption, birds will continue to lose body weight. Gross necropsies commonly show birds to be malnourished, with atrophy of breast muscle and little interior body fat. Caseous nodules in the liver, spleen, intestines, and bone marrow can range in size from white to gray. Mycobacterium rods can enter the gut lumen and contaminate feces through intestinal wall ulceration caused by tubercles. Histologic examination of damaged tissues provides a quick and accurate diagnosis. Tubercles are isolated granulomas that can infiltrate the intestinal wall at any layer. Granulomas often have necrotic cores and are isolated from surrounding tissue by fibrous connective tissue. Acid-fast bacilli can be seen in the necrotic core and cytoplasm of epithelioid macrophages within the granuloma.
Staphylococcus infection
Staphylococcus, a gram-positive bacteria, is commonly found in mucous membranes and skin of chickens. Staphylococcus possesses a clumping component that converts fibrinogen, a soluble blood protein, into insoluble fibrin molecules. The bacterium is buried in fibrin clots from phagocytic cells. Protein A attaches to a part (FC) of immunoglobulin, inhibiting phagocytosis. While Staphylococcus aureus is the most prevalent cause of avian staphylococcosis, additional species such as S. epidermidis, S. agnetis, S. cohnii, S. simulans, and S. hyicus have also been found in skeletal lesions (Zhu 1998). Eid et al. (2019) identified S. aureus from sick ducks (12.2%) (Eid, Algammal et al. 2019).However, Lebdah et al. (2015) discovered that S. aureus isolates were susceptible to cefotaxim, ciprofloxacin, enrofloxacin, and sulpha trimethoprim (Lebdah, Youssef et al. 2015).
Cholera infection
Fowl cholera is a genuine bacterial disease affecting chickens, turkeys, ducks, and game birds.
Bacteremia and endotoxemia are generally the causes of death, particularly in acute instances. Birds may die without clinical symptoms, but may exhibit depression, cyanosis, and diarrhea.
Birds that survive may have persistent infections in joints, wattles, footpads, sinuses, middle ear, bones, sternal bursae, and other areas. Fowl cholera is caused by the Pasteurella multocida bacteria (Boulianne, Blackall et al. 2020). Pasteurella multocida is a Gram-negative, nonmotile rod or coccobacillus with varying virulence according on strain. Fowl cholera, like mycobacteriosis and coligranuloma, is often found in adult or young adult birds, unlike other bacterial infections included in this study. Gross lesions are characterized by septicemia and include hemorrhages and tiny necrotic foci throughout the liver and viscera. Turkeys frequently exhibit necrofibrinous pneumonia (Porter Jr 1998). The small intestine may experience significant mucus collection and congestion. Histopathologic examination of the gut may only demonstrate considerable mucosal congestion.
Bordetella infection
Turkey coryza is a severe respiratory infection caused by Bordetella avium, often known as bordetellosis. The disease affects turkeys of all ages, causing substantial morbidity (up to 100%) but moderate mortality rates (El-Ghany and Research 2022). Turkey coryza symptoms include respiratory-related, including nose and ocular discharge, sneezing, coughing, and submaxillary edema. The sickness typically lasts 2 to 4 weeks (Jackwood and Saif 2008). Postmortem examinations typically reveal air-saculitis, pneumonia, and tracheitis bronchitis, as well as exudates in the nasal cavity and trachea. Internal organs are usually unaffected, unless there are secondary infections. B. avium was detected in Egyptian turkey herds. Two strains were identified from 21 turkey farms in 5 Egyptian locations. PCR was used to detect B. avium infection in Egyptian turkeys of different ages. The total PCR-confirmed prevalence of B. avium was 22.95%. The detected B. avium strains included virulence-associated genes responsible for colonization in turkeys’ respiratory tracts, including B. avium virulence gene (100%), fimbriae (71.14%), and filamentous hemagglutinin (85.68%) (Eldin, Abd-El Samie et al. 2020).
Salmonella infection
Salmonella has about 2,000 serotypes identified by somatic (O), flagellar (H), and capsular (Vi) antigens. Salmonella is a Gram-negative rod that is 2 to 3 mm long and does not sporulate. Salmonella often colonizes the digestive system and spreads through feces. Salmonella infections can cause systemic symptoms, including gastrointestinal lesions. Antibiotic medication, feed restriction, and coccidial illness can disrupt the natural intestinal microbiota, making the host more susceptible to Salmonella infections (Broom 2021). Salmonella-induced intestinal lesions in chicken are linked to three diseases: pullorum disease, fowl typhoid, and paratyphoid infections. Enteric lesions are commonly seen in the cecum, making it the ideal location for bacterial isolation in these disorders. The paratyphoid group, which is defined as Salmonella serotypes other than S. pullorum, S. gallinarum, and S. arizona, contains the majority of Salmonella serotypes. Numerous host species are susceptible to infection by the motile paratyphoid Salmonella.
Salmonella typhimurium, S. enteritidis, S. montevideo, and S. heidelberg are a few types of paratyphoid Salmonella (Hafez 2013). Over the past 10 years, parapyphoid infections have drawn a lot of attention since chickens are a major source of Salmonella, which may cause foodborne sickness in people.
Conclusion
Effective prevention and control strategies are crucial in managing bacterial diseases and disorders in poultry. This includes maintaining strict biosecurity protocols, implementing vaccination programs, ensuring proper sanitation and hygiene practices, and promptly identifying and treating affected birds. Collaboration between poultry producers, veterinarians, and regulatory authorities is essential to mitigate the impact of these diseases on both animal health and food safety. By implementing comprehensive disease management strategies, poultry producers can minimize losses, safeguard animal welfare, and maintain a sustainable and profitable poultry industry.
References
Amare, A., A. M. Amin, A. Shiferaw, S. Nazir and H. J. A.-E. J. o. S. R. Negussie (2013). “Yolk sac infection (omphalitis) in Kombolcha poultry farm, Ethiopia.” 8(1): 10-14.
Boulianne, M., P. J. Blackall, C. L. Hofacre, J. A. Ruiz, T. S. Sandhu, H. M. Hafez, R. P. Chin, K. B. Register and M. W. J. D. o. p. Jackwood (2020). “Pasteurellosis and other respiratory bacterial infections.” 831-889.
Broom, L. J. J. W. s. P. S. J. (2021). “Evidence-based consideration of dietary ‘alternatives’ to anticoccidial drugs to help control poultry coccidial infections.” 77(1): 43-54.
Chaidez‐Ibarra, M. A., D. Z. Velazquez, I. Enriquez‐Verdugo, N. Castro Del Campo, M. A. Rodriguez‐Gaxiola, A. Montero‐Pardo, D. Diaz, S. M. J. T. Gaxiola and e. diseases (2022). “Pooled molecular occurrence of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry: A systematic review and meta‐analysis.” 69(5): 2499-2511.
Crump, J. A., P. M. Griffin and F. J. J. C. I. D. Angulo (2002). “Bacterial contamination of animal feed and its relationship to human foodborne illness.” 35(7): 859-865.
Deblais, L., D. Kathayat, Y. A. Helmy, G. Closs and G. J. A. H. R. R. Rajashekara (2020). “Translating ‘big data’: better understanding of host-pathogen interactions to control bacterial foodborne pathogens in poultry.” 21(1): 15-35.
Dewey, C., K. Bottoms, N. Carter, K. J. J. o. S. H. Richardson and Production (2014). “A qualitative study to identify potential biosecurity risks associated with feed delivery.” 22(5): 232-243.
Eid, H. M., A. M. Algammal, W. K. Elfeil, F. M. Youssef, S. M. Harb and E. M. J. V. W. Abd-Allah (2019). “Prevalence, molecular typing, and antimicrobial resistance of bacterial pathogens isolated from ducks.” 12(5): 677.
El-Ghany, W. A. A. J. O. J. o. A. and F. Research (2022). “Avian bordetellosis: a significant bacterial respiratory disease of turkeys (Meleagris gallopavo).” 12(3): 103-110.
Eldin, W. F. S., L. K. Abd-El Samie, W. S. Darwish, Y. H. A. J. T. a. h. Elewa and production (2020). “Prevalence, virulence attributes, and antibiogram of Bordetella avium isolated from turkeys in Egypt.” 52(1): 397-405.
Elmberg, J., C. Berg, H. Lerner, J. Waldenström, R. J. I. e. Hessel and epidemiology (2017). “Potential disease transmission from wild geese and swans to livestock, poultry and humans: a review of the scientific literature from a One Health perspective.” 7(1): 1300450.
Fancher, C. A., L. Zhang, A. S. Kiess, P. A. Adhikari, T. T. Dinh and A. T. J. M. Sukumaran (2020). “Avian pathogenic Escherichia coli and Clostridium perfringens: Challenges in no antibiotics ever broiler production and potential solutions.” 8(10): 1533.
Ferens, W. A., C. J. J. F. p. Hovde and disease (2011). “Escherichia coli O157: H7: animal reservoir and sources of human infection.” 8(4): 465-487.
Hafez, H. M. J. S. i. d. a. (2013). “10 Salmonella infections in Turkeys.” 193.
Hu, J., D. J. A. Afayibo, B. Zhang, H. Zhu, L. Yao, W. Guo, X. Wang, Z. Wang, D. Wang and H. J. F. i. M. Peng (2022). “Characteristics, pathogenic mechanism, zoonotic potential, drug resistance, and prevention of avian pathogenic Escherichia coli (APEC).” 13: 1049391.
Jackwood, M. W. and Y. J. D. o. P. t. e. B. P. Saif, Ames (2008). “Bordetellosis (turkey coryza).” 774-788.
Karunarathna, R., S. Popowich, M. Wawryk, B. Chow-Lockerbie, K. A. Ahmed, C. Yu, M. Liu, K. Goonewardene, T. Gunawardana and S. J. A. d. Kurukulasuriya (2017). “Increased Incidence of enterococcal infection in nonviable broiler chicken embryos in Western Canadian hatcheries as detected by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry.” 61(4): 472-480.
Kemmett, K., N. Williams, G. Chaloner, S. Humphrey, P. Wigley and T. J. A. p. Humphrey (2014). “The contribution of systemic Escherichia coli infection to the early mortalities of commercial broiler chickens.” 43(1): 37-42.
Lebdah, M. A., F. M. Youssef and E.-E. A. J. Z. V. J. Elwan (2015). “Bacterial leg infections in broiler chickens.” 43(3): 179-188.
Lee, A. (2022). Investigating the prevalence of antimicrobial resistance and virulence factors of Escherichia coli isolated from caecum in broiler chickens, University of Reading.
Leighton, R. E. (2018). Escherichia coli, Poultry, and Precipitation: A Watershed Story, The University of North Carolina at Chapel Hill.
Luo, R., C. Fan, G. Jiang, F. Hu, L. Wang, Q. Guo, M. Zou, Y. Wang, T. Wang and Y. J. B. P. S. Sun (2023). “Andrographolide restored production performances and serum biochemical indexes and attenuated organs damage in Mycoplasma gallisepticum-infected broilers.” 64(2): 164-175.
Momba, M. N., V. K. Malakate, J. J. J. o. w. Theron and health (2006). “Abundance of pathogenic Escherichia coli, Salmonella typhimurium and Vibrio cholerae in Nkonkobe drinking water sources.” 4(3): 289-296.
Njuguna, K. W. (2007). Evaluation of rice milling by-products from Mwea irrigation Scheme in layer chicken diets, University of Nairobi.
Olsen, R., C. Frantzen, H. Christensen and M. J. A. d. Bisgaard (2012). “An investigation on first-week mortality in layers.” 56(1): 51-57.
Porter Jr, R. E. J. P. S. (1998). “Bacterial enteritides of poultry.” 77(8): 1159-1165.
Saxena, T., P. Kaushik, M. K. J. D. m. Mohan and i. disease (2015). “Prevalence of E. coli O157: H7 in water sources: an overview on associated diseases, outbreaks and detection methods.” 82(3): 249-264.
Shah, D. H., N. C. Paul, W. C. Sischo, R. Crespo and J. J. P. S. Guard (2017). “Population dynamics and antimicrobial resistance of the most prevalent poultry-associated Salmonella serotypes.” 96(3): 687-702.
Swelum, A. A., A. R. Elbestawy, M. T. El-Saadony, E. O. Hussein, R. Alhotan, G. M. Suliman, A. E. Taha, H. Ba-Awadh, K. A. El-Tarabily and M. E. J. P. s. Abd El-Hack (2021). “Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: an updated overview.” 100(5): 101039.
Zhu, X. (1998). The pathogenesis of staphylococcosis in poultry, Purdue University.