Collection and Submission of Laboratory Samples for Disease Diagnosis Dr.Parvinder Kaur Lubana , Deputy director A.H. ( NRDDL ), Jalandhar, Punjab
Collection of samples plays most important role in exact diagnosis and confirmation of the disease. Biological and non biological samples have to be collected and sent to the laboratory to arrive at a correct diagnosis. But apart from this, the most important is appropriate sampling, their preservation; its proper labeling and its transportation to laboratory in appropriate environment. There are specific methods for collection of specific samples for respective diseases.
Some of the measures to be adopted for proper collection of samples are:
- Correct sampling
- Correct preservation
- Correct Labelling and Identification:
- Owner’s name &Address
- Species
- Breed
- Sex
- Age
- A detailed case history should include:
- Clinical signs
- Gross appearance (including size and location) of the lesion(s)
- Previous treatment (if any)
- number of animals at risk and any mortality in the group
- Mention type of sample submitted
- Tentative diagnosis
- Clinician’s Name and Contact number
General consideration for submission of samples
- If possible, Sample should be collected before administration of any treatment.
- Samples should be collected from the affected site as early as possible following onset of clinical symptoms.
- Samples should be collected from living and recently dead animals.
- Samples should be collected from clinical cases, in-contact animals and healthy animals.
- Sample should be appropriate for the intended purpose, adequate in number and amount to provide statistically valid results.
- Samples should be collected without environmental contamination and avoid cross contamination between samples.
- Sample must be carefully packed, labelled and transmitted to the laboratory.
- Samples should be labeled with permanent marker. Use of gel pen should be avoided.
- A duplicate sample should always be kept in vetro-legal cases.
- For Bacteriological and Virological examination the material should be transported in ice.
- For paired serum samples, one sample should be collected at onset of disease and second sample should be collected at least 14-21 days after first
Collection of various Samples
Blood Sample
In Cattle, Horses, Sheep &Goat, Blood should be collected from Juglar vein.
In Dogs &Cats: Cephalic vein or Saphenousvein.
Birds: Wing vein or Comb.
Pigs: Ear vein or Juglar vein
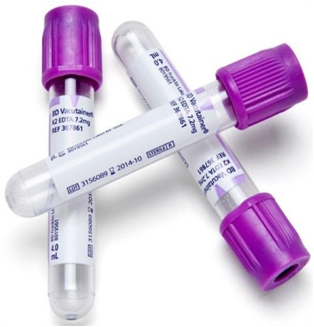
Blood sample should be collected in clean dry vials/tubes. (Figure 1)
- Hematology/culture/direct examination: Use anticoagulant (EDTA coated vacutainer be used). Heparin is also used.
- Serology:Clotted blood (Serum vacutainers)
- Parasitology:In EDTA vial or wet blood film or thin blood smear on glass slide.
Faecal Sample
- For Parasitic or Microbiological investigation.
- Fresh faecal sample (2-5g) should be collected from rectum. (Figure 2)
- If transport time is likely to be longer than 24hrs, the sample should be sent on ice or refrigerated to prevent the hatching of parasitic eggs.
- Faeces are best stored and transported at 4˚C and 5% formalin.
Urine Sample
- Urinalysis, Microbiological investigation.
- Fresh Urine sample should be collected aseptically by midstream or catheter. (Figure 3)
- Microbiological examination: Avoid contamination, refrigerate immediately and transported to lab within 1hr.
- Fresh urine sample within 20 minutes of collection should be submitted to lab to examine Leptospira motility, otherwise to study Leptospira motility submit 20ml urine sample preserved with 1.5ml of 10% formalin.
Skin Sample
- Burrowing Mites/Mange: Deep skin scraping using scalpel blade. (Figure 4)
- Surface-feeding mite/lice or Fungal Infection: Plucked hair or superficial skin scrapings
- Add few drops of 10% KOH.
- Submitted in cotton plugged test tube or paper envelop.
Milk Sample
- Milk should be collected aseptically from each affected quarter and marked properly as from LF, LH, RF, RH, etc. (Figure 5)
- The initial stream of milk should be discarded.
- Bacteriological examination: Milk sample should be refrigerated and sent immediately to laboratory.
Rumen Liquor
- Collected by stomach tube, rumen fistula and by hypodermic needle (6 inch) from Paralumbar Fossa. (Figure 6)
- Mercuric chloride @ 1mg/5ml of rumen liquor can be used as preservative.
Lymph Node Smear
- Inject 1-2ml of sterile PBS to Prescapular lymph node, rub it and suck the fluid from the same. Prepare smear and stain it for demonstration of Theileria. (Figure 7)
Pus, Sputum, Nasal, Throat, Lacrimal secretions
- These are collected on sterile cotton swabs. (Figure 8)
- Swab should be allowed to remain in contact with the secretions for upto 1 minute, then place in transport media and send to laboratory at 4˚C.
Genital tract and Semen
- Vaginal mucus swabs: For diagnosing the bacterial cause of infertility. In case of bitches, to detect stage of heat.
- Semen: Best obtained using an Artificial Vagina for examining quality of semen and in preventing spread of sexually transmitted diseases.
Preputial washings
- About 50ml of sterile PBS (pH 7.2-7.4) inserted in prepuce, massaged and collected in sterile containers
- For demonstration and culturing of Campylobacterosis and Trichomonosis.
Brain Sample
- BSE surveillance: Complete Brain (including Brain Stem) to be sent on ice.
- Rabies: Send complete Brain on ice. (Figure 9)
Sample collection at Post-Mortem
- Tissues collected either via biopsy or during necropsy. (Figure 10)
- Each piece of tissue should be placed in a fully labelled separate plastic bag or screw capped jar.
- Samples collected for histology should never be >1 cm thick (preferably 5–7 mm) and must be placed immediately into ≥10 times their volume of 10% formalin to ensure adequate fixation.
- Because the GI mucosa decomposes rapidly, short sections of Gut collected at necropsy should be opened lengthwise to allow adequate fixation.
- Tissue samples collected at necropsy should include some of the apparently normal surrounding tissue.
- Excisional biopsies of small tumors (<1.5 cm) may be cut in half. Larger tumors may be sliced like bread so that formalin can penetrate to the face of each slice.
- Autolyzed tissues are generally useless for Histopathologic examination.
- For microbiological samples care should be taken to avoid any contamination and samples should be refrigerated until shipped.
- Send Postmortem report along with samples.
Sampling in Poisoning cases
- The samples for toxicological investigations be sent preferably on ice without preservative
- Desirable Sample collection for various diseases
| Condition | Ante mortem sample | Post mortem sample |
| Haemorrhagic Septicaemia | Smear from ear vein, smear from fluid of swelling of throat, blood | Lungs, intestine, spleen, liver, heart blood |
| Brucellosis | Blood & serum, uterine fluid, milk of mother in sterile tube | Stomach content of aborted fetus, liver, placenta |
| Tuberculosis | Sputum, milk, faeces | Lungs, lymph glands showing nodular lesions, intestine, mesentric lymph glands |
| Glanders | Serum, Nasal discharge, pus from skin lesions | Lungs and superficial lymph nodes |
| Lumpy Skin Disease | Blood in EDTA, serum, Skin scab | Lung, liver, kidney or organs showing nodular lesions. |
| Foot & Mouth Disease | Vesicular fluid, tongue epithelium, hoof lesions sample, etc | Vesicular fluid, tongue, heart tissue, hoof lesions sample |
| African Swine Fever | Blood in EDTA, Serum | Lymph node, tonsils, spleen, liver, lungs |
| Rabies | Saliva, eye discharge | Brain |
| Bovine viral Diarrhea | Nasal discharge, ocular discharge, blood | Spleen, lymph nodes, liver, blood |
| Mastitis | Milk and pus from affected quarter | —— |
Packaging and Transport of Samples
- Make sure container is leak proof, properly sealed and is not contaminated.
- If specimen is collected in syringe, remove the needle and replace it with cap prior to transport.
- The specimen just after collection is kept in appropriate preservative and sent to laboratory over ice preferably in thermos flask or insulated box.
- Box should be properly labelled.
- If a Zoonotic disease is suspected, this should also be clearly indicated on the submission form to alert laboratory personnel.
- Bacteriological samples should be refrigerated and not frozen. It should not be contaminated. Appropriate transport media should be used.
- For virological samples 50% buffered Glycerine saline is general preservative.
- Swabs for virus isolation should be placed into tissue culture fluid or isolation media.
- All parasitological samples should be dispatched in 5-10% formalin or 70% alcohol.
- Dry ice may be used if journey is longer. It is recommended that samples be sent to diagnostic laboratory without delay.
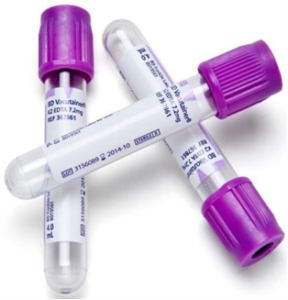


Fig.1: EDTA vacutainer, serum vacutainer, serum vials
Fig. 2: Faecal sample collected in clean
plastic containers
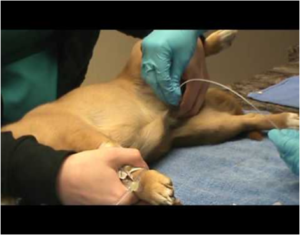
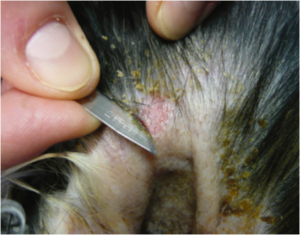
Fig.3 Urine collection using catheter in dogs
Fig. 4 Collection of skin scrapings using scalpel blade

Fig.5: Milk sample collected aspectically from
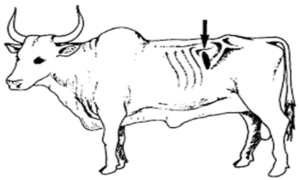
Fig. 6: Site of Paralumbar fossa for collection of rumen
affected quarter. liquor.

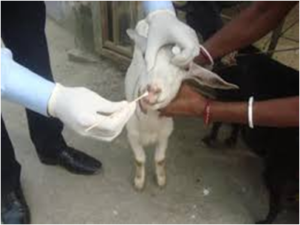
Fig. 8 sterile cotton swab for collection of various secretions
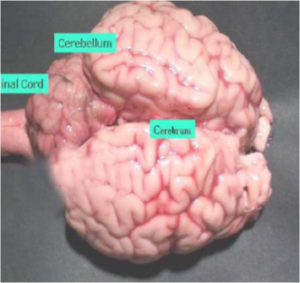
Fig.9: Brain sample
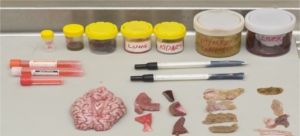

Fig.10: Various tissue samples collected during post-mortem Fig.11: Proper packing of samples maintaining
cold chain