Foot and Mouth Disease-A most Contagious Disease of Cloven-Footed Animals
Das T1 and Das NK2
Scientist, ICAR-DFMD, Bhubaneswar
BVO, Bisoi, Mayurbhanj, FARD, Government of Odisha
Corresponding author E mail id: tarenisahoo@gmail.com
Foot and mouth disease (FMD) is one of the most contagious transboundary, devastating and debilitating viral diseases of cloven footed domestic and wild animals having major global health significance. It is a highly mutable virus with seven serotypes and many subtypes within a serotype. The most common serotypes prevalent worldwide is serotype O. It causes high morbidity in adult animals and high mortality in younger calves and piglets due to myocarditis causing severe production losses and severe damage to economy of our country. Vaccination is one of important key preventive measures to control FMDV. But due to lack of sufficient cross protection ability of present vaccine between different serotypes and subtypes, a regular vaccine matching test has been carried out.
Aetiology
FMD is ranked as 1st among notifiable list A disease of animals (Mekonen et al., 2011). It is caused by Apthovirus of the family Picornaviridae. Hieronymous Fracastorius had given 1st written description of FMD in 1514 in Italy. In 1898, Friedrich Loffler for the first time discovered the filterable causative agent of FMD (Lefevere et al., 2010). It is having seven antigenetically different serotypes based on antigenecity of capsid coating region and above 60 subtypes. In 1922, Vallee and Carre showed the existence of two serotypes (Serotype O from France and Serotype A from Germany). Then in 1926, Waldmann and Trautwein reported existence of serotype C along with serotype O and A. In 1940, three serotypes (SAT-1, 2 and 3) were discovered from South Africa (Brooksby, 1958) and in 1951 and 1952, Asia-1 was recognised from India (Dhanda et al., 1957).
FMDV is a roughly spherical, non enveloped single stranded positive sense RNA virus and is 26 nm in diameter (Alexandersen et al., 2003). The 8.5 kb RNA genome is surrounded by capsid consisting of 60 copies of each structural protein (VP-1, 2, 3, 4). The genome consists of 5’ UTR, ORF and 3’ UTR. The ORF site is divided L, P1, P2 and P3 region. The L site encodes for Leader proteinase, P1 encodes for VP4, VP2, VP3, and VP1. The VP1 is most immunogenic. P2 encodes for three non structural proteins (NSP) like 2A, 2B and 2C. P3 encodes for four NSP such as 3A, 3B, 3C and 3D. The 3’ UTR is important for virus replication. The VP1 region contains two important sites like C- terminus and G-H loop containing RGD motifs which are essential for antigenicity and receptor binding. FMDV binds to two classes of receptors like integrins and heparin sulphate proteoglycan. However, integrin αvβ6 heterodimer receptor whose expression is restricted to epithelial cells is expected to be a principal receptor for FMDV (Rieder et al., 1996). FMDV is highly mutable virus due to lack of proof-reading activity of RNA polymerase. The virus is very much environmental resistant and inactivated at temperature > 56°C and PH beyond 6-9. Sodium hydroxide and sodium carbonate are effective disinfectant. Lipid solvents are ineffective.
Epidemiology
FMD occurs mainly in cloven footed animals. Among domestic animals, the disease is more serious in cattle and pig. About 70 wild life species can get infection (Verma et al., 2012). Apart from cloven footed animals, hedgehogs, armadillos, capybaras, guinea pig, rat and mice are susceptible. The morbidity rate depends on species, age, sex and immunity status. The morbidity rate of animals in outbreaks can approach 100% but the case fatality rate is very low i.e., 2% in adults and 20% in young ones (Radostits et al., 2007). Mortality in calves is usually up to 50% due to cardiac involvement, malnutrition and secondary bacterial infection (MacLauchlan and Dubovi, 2011). About 70 countries are officially recognised as FMD free by OIE, but still India along with 100 other countries are considered as endemic or sporadic zones (OIE, 2009). Countries like America, New Zealand, Australia and most of the Europe are free from FMD. In South America and Europe, serotype O, A and C are prevalent; in Africa, all serotypes except Asia-1 are found and in Asia; O, A, C and Asia-1 are prevalent. The most common serotypes prevalent worldwide is serotype O. Serotype O is responsible for 80% FMD outbreaks followed by Asia-1 and serotype A. Serotype-C is considered as extinct as it has not been detected since 2005 (Paton et al., 2021).
In India, it is a major threat to the livestock sector as it has world’s largest susceptible livestock population (535.78 million) of which cattle and buffalo constitute 302.34 million (DAHD &F, 2019). Livestock production contributes 4.11% to national gross domestic product and in India 20.5 million people depend on livestock for their livelihood. (https://vikaspedia.in/agriculture/livestock/role-of-livestock-in-indian-economy). Among various diseases affecting livestock production, FMD is one of the most important. About 252 FMD incidences were reported in India during the year 2015-2016 (DFMD, Mukteswar, Annual report, 2015-2016). FMD has severe economic significance as it causes both direct and indirect losses. The direct losses include loss of milk production and draught power, lower weight gain, delay in sale or no sale of animal products, fertility problems and death of animals. In India, direct losses costing Rs. 20,000 crores have been reported (Venkataramanan, 2006). The indirect losses include due to additional cost for vaccine, diagnostics, therapeutics, due to culling of animals and due to denied access to market etc. (Admassu et al., 2015).
Transmission
After infection, animal shed virus in all excretions and secretions of the body such as saliva, tear, nasal fluid, milk, semen, urine and faeces. The animal may shed virus four days prior to onset of symptoms up to years after recovery. Transmission occurs through inhalation of aerosolised virus, ingestion of contaminated food, direct inoculation and contact with infected animals, contaminated fomites and fodder, sexual contact in African Buffaloes etc. Also, hair and wool of infected animals, foot wear and clothing of animal handlers, vehicle tyres, animal products, mechanical carriage and wind can transmit virus. In favourable environmental conditions like high humidity >55%, moderate wind and low temperature, large emission of virus most likely from pigs in acute stage of infection; virus can spread up to 250 kms through wind causing infection (Donaldson et al., 2001). Airborne transmission is a low probability and high consequence event. Under favourable environmental condition, the emitted infected aerosol can travel long distances in the air and infect susceptible livestock like cattle beyond the imposed quarantine zones (Brown et al., 2022). The virus may persist for years in infected premises and for 10-12 weeks on clothes and feeds (Radostits et al., 2007). The virus can survive for 14 days in dry faecal materials in summer, up to 6 months in slurry in winter and 39 days in urine in winter. Some animals after infection act as carrier shedding the virus for long periods. In cattle it persists in oropharynx for one year, in sheep it persists in tonsil for 6-9 months, in goats up to 4 months and in African Buffaloes up to 24 years. Horizontal transmission of FMD virus occurred more easily between the same species animals as compared to different species animals (Fukai et al., 2020). Also, vertical transmission of FMDV and associated abortion was also reported in cattle from natural infection (Ranjan et al., 2016).
Pathology
The incubation period of FMD varies from 18 hours to 12 days. The respiratory system is the important route of infection. After infection, virus primarily replicates in the pharynx, then replicate in the lymphoid tissue of pharynx, oral mucous membrane and tongue epithelium. The virus multiplies in stratum spinosum layer causing cytolysis and ballooning degeneration. Virus also spread to the site of secondary multiplication in lymph nodes, epithelial tissues in and around mouth, feet and teat (Lefevere et al., 2010). Virus appears in the body fluids few days before appearance of clinical signs. Lesions are grossly visible in those areas which are subjected to mechanical trauma or physiological stress. Due to secondary bacterial complication mastitis and laminitis may develop. In young animals and aborted foetus, it causes myocardial degeneration and necrosis resulting in death.
The clinical signs are more severe in cattle and pigs (Rufael, 2006). The clinical signs usually appear 3-5 days after infection. It is characterized by anorexia, fever, vesicles on the feet, interdigital space, coronary band, heels, lip, tongue, palate and teat (Alexandersen et al., 2003); rarely on external genitalia. Vesicles may enlarge and rupture exposing painful raw erosions and ulcers (Fig. 1). The animal shows depression, excessive salivation, lameness and abortions due to high body temperature, loss of condition, infertility, mastitis and reduced milk production due to secondary bacterial infection. Very young animals may die before onset of vesicular lesions due to necrotizing myocarditis. In pig, sudden onset of severe lameness is the commonest finding. In sheep and goat, very mild clinical signs are observed.
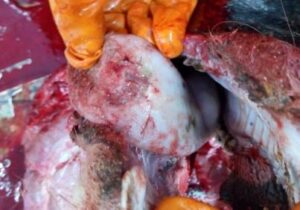
The lesions in FMD mainly consist of fluid filled vesicles / bullae in the mouth, on the feet and udder. Later on, vesicles / bullae ruptured leaving raw eroded lesions or ulcers. The lesions are formed due to ballooning degeneration in the stratum spinosum layer. Vesicle formation in cattle may occur in multiple organs like teat, udder, coronary band, interdigital space, external genitalia, fore stomach etc. The mucosa of abomasum and small intestine may show pin point haemorrhage and oedema. The mucosa of large intestine may be hyperaemic. In young animal, the myocardium of the ventricular wall appears stripped due to grey or yellow patches of tissue interspaced with normal tissue giving typical tiger heart appearance (Fig. 2). The grey and yellow patches are mainly due to myocardial degeneration and necrosis along with leukocyte infiltration (Vegad and Katiyar, 2008).
 |
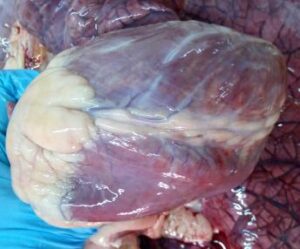 |
| Fig.1. One large ulcer on the tip of tongue in FMDV infected cattle | Fig.2. Tigered heart appearance of myocardium in a FMD affected calf |
Diagnosis
The diagnosis is mainly based on clinical signs, PM findings and laboratory diagnosis like virus isolation, serological and molecular techniques. For laboratory diagnosis, epithelium or vesicular fluid is the tissue of choice. Virus isolation can be carried out in primary bovine thyroid cells, primary pig, calf or lamb kidney cells, BHK-21 or IB-RS-2 cells or in unweaned mice.
The viral antigens can be detected by CFT and ELISA. The virus antibody can be detected by VNT, AGID, Solid phase competitive ELISA, liquid phase blocking ELISA, enzyme linked immune electro transfer blot assay (Hughes et al., 2002) etc. For differentiation of infected from vaccinated animals (DIVA), NSP based ELISA tests are used. A chromate graphic test like pen side has been developed for rapid detection of serum antibody (Chen et al., 2009 and Sammin et al., 2010). For rapid detection of nucleic acid, RT-PCR, LD-PCR, nested RT-PCR, real time RT-PCR, NASBA, RT-LAMP (Dukes et al., 2006; Ding et al., 2013 and Dhama et al., 2014), gold nano particle immuno PCR (Ding et al., 2011), multiplex PCR, one step SYBR green based real time RT-PCR (Biswal et al., 2022) have been developed.
Prevention and control
As FMD is one of economically important transboundary disease, control measures are implemented both at national and international level depending upon disease status whether the country is free from disease or is subject to sporadic outbreaks or is endemic to FMD. In FMD free countries, there are stringent regulation on import of animals and animal products from FMD countries. Quarantine, vaccination and stamping out policy along with disinfecting premises and intensified surveillance followed during the outbreak to prevent further spread of virus (Hirsh et al., 2004). In endemic areas, high yielding animals are protected through vaccination followed by seromonitering and control on animal movement.
Vaccination is the most important strategy to control FMD. The conventional vaccine protects animals and decreases FMDV spread to adjoining areas. Recently available vaccine has disadvantages like production of carrier status which shed viruses up to many years and also these vaccines cannot protect from virus replication or infection. Timing of vaccination, availability of quality vaccine, incorporation of multiple serotypes/ strains of FMDV circulating in the particular area, maintenance of cold chain, vaccination of susceptible population is important for controlling FMD. The currently available inactivated vaccine provide protection only for 4 to 6 months and require booster bi annually. In India, most of the FMD vaccines are BHK-21 cell culture based inactivated trivalent vaccine containing O, A, Asia-1 serotypes. Some commercially available vaccines are Raksha Ovac (Indian immunologicals), Bovilis clovax (Intervet), Bio vet FMD vaccine etc. But due to various limitations such as lack of cross protection between serotypes/ strains, risk of live virus escapes from laboratory, limited shelf life and repeated dosing, development of new alternative vaccine like DNA vaccine, synthetic peptide vaccine, epitope-based vaccine, chimeric vaccines like VP1 and IL-2 vaccine, FMDV VP1 and swine IgG, cocktail vaccine based on VP1 and 3D genes are on progress (Chakraborty et al., 2014). The antigenic variation that arises due to emergence of new lineage and sub lineage of FMDV creates problems in cross protection. So regular epidemiological survey regarding current circulating viruses and vaccine matching test should be carried out which is helpful in selecting current vaccine strain and control of FMD (Upadhyaya et al., 2014).
Progressive control pathway for FMD was established with the aim to determine national progress in FMD control and to develop national and international action plans and support to reduce circulation of virus and to mitigate the impact of FMD (Jamal and Belsham, 2013). In India, two programmes are operational in the country like FMD control programme (FMDCP) and assistance to states for control of animal disease (ASCAD). ASCAD is GOI and state government programme implemented in non FMD states. Here about 85 million animals were covered. The FMD CP was launched in India in 2003-2004 in 54 districts covering 30 million cattle and buffalo with an objective to create and expand FMD free zones. Later in 11th plan, additional 167 districts and 80 to 90 million animals were covered under FMD vaccination. Till now it covers 351 districts in 13 states and 6 UT (28). Due to robust implementation of FMDCP, disease occurrence has been drastically reduced. However, 16 states and one UT, which are not covered under intensive FMD vaccination. Now it will be covered under Rastriya Krishi Vikash Yojna (RKVY) during 2016-2017 in order to conceive FMD Mukt Bharat (Press information bureau, Govt. of India, Ministry of Agriculture, 09- August-2016, FMD Mukt Bharat in next few years). Recently, in 2019, National Animal Disease Control Programme (NADCP) is launched by Central Government with the aim of controlling FMD by 2025 and eradicating FMD by 2030 by 100% vaccinating all the susceptible livestock population in India including cattle, buffalo, sheep, goat and pigs at 6 months interval (https://dahd.nic.in/national-animal-disease-control-programme).
Conclusion
FMD is one of the greatest obstacles for the development of animal husbandry sector and the livestock economy thereof. For this purpose, FMD control is essential. The control of FMD is very difficult due presence of multiple serotypes and subtypes and also due to presence of carrier states. Continuous efforts have been made to control FMD through sero-surveillance, effective vaccination programme, strict biosecurity and biosafety measures, educating farmers and by stamping out whenever possible. Phenotypic and genetic characterization with molecular cloning is carried out to develop effective vaccine and diagnostics. So, all the above measures are important keys for successful control of FMD.
References
Admassu B, Getnet K, Shite A and Mohammed S. (2015). Review on Foot and Mouth Disease: Distribution and Economic Significance, Academic Journal of Animal Diseases 4(3): 160-169.
Alexandersen S, Zhang Z, Donaldson AI and Garland AJM (2003). Review: The Pathogenesis and diagnosis of Foot-and-Mouth Disease. J. Comp. Pathol. 129: 1–36
Biswal, J.K., Jena, B.R., Ali, S.Z., Ranjan, R., Mohapatra, J.K. and Singh, R.P. (2022). One-step SYBR green-based real-time RT-PCR assay for detection of foot-and-mouth disease virus circulating in India. Virus Genes, 58(2), pp.113-121.
Brooksby JB (1958). The virus of foot-and-mouth disease. Adv Virus Res 5:1–37
Brown, E., Nelson, N., Gubbins, S., & Colenutt, C. (2022). Airborne Transmission of Foot-and-Mouth Disease Virus: A Review of Past and Present Perspectives. Viruses, 14(5), 1009.
Chakraborty S, Naveen Kumar, Dhama K, Verma AK, Tiwari R, Amit Kumar, Kapoor S, Singh SV (2014). Foot and mouth disease, an economically important disease of animals, Advances in Animal and Veterinary Sciences. 2 (2S): 1 – 18
Chen TH, Pan CH, Jong MH, Lin HM, Huang YL, Hsiung KP, Chao PH and Lee F (2009). Development of a chromatographic strip assay for detection of porcine antibodies to 3ABC non-structural protein of Foot-and-Mouth Disease Virus serotype O. J. Vet. Med. Sci. 71(6): 703–708
DFMD, Mukteswar, Annual report (2015-2016).
Dhama K, Karthik K, Chakraborty S, Tiwari R, Kapoor S, Verma AK and Thomas P (2014). Loop-mediated isothermal amplification of DNA (LAMP)-a new diagnostic tool lights the world of diagnosis of animal and human pathogen: A review. Pak. J. Biol. Sci. 17(2): 151-166
Dhanda MR, Gopalakrishnan VR, Dhillon HS (1957). Note on the occurrence of atypical strains of foot-and-mouth diseases virus in India. Ind J Vet Sci 27:79–84
Ding YZ, Chen HT, Zhang J, Zhou JH, Ma LN, Zhang L, Gu Y and Liu YS (2013). An overview of control strategy and diagnostic technology for foot-and-mouth disease in China. Virol. J. 10(1): 78
Ding YZ, Liu YS, Zhou JH, Chen HT, Wei G, Ma LN and Zhang J (2011). A highly sensitive detection for foot-and-mouth disease virus by gold nanoparticle improved immuno-PCR. Virol. J. 8: 148
Donaldson AI, Hearps A and Alexandersen S (2001). Evaluation of a portable, ‘real-time’ PCR machine for FMD diagnosis. Vet. Rec. 149: 430.
Dukes JP, King DP and Alexandersen S (2006). Novel reverse transcription loop-mediated isothermal amplification for rapid detection of foot-and-mouth disease virus. Arch. Virol. 151(6): 1093-1106
Fukai, K., Nishi, T., Morioka, K., Yamada, M., Yoshida, K., & Yamakawa, M. (2020). Horizontal transmission of foot‐and‐mouth disease virus O/JPN/2010 among different animal species by direct contact. Transboundary and emerging diseases, 67(1), 223-233.
Hirsh, C.D., N.J. MacLauchlan and R.L. Walner, (2004). Veterinary Microbiology. 2nd ed. Black Well Science, pp: 341.
Hughes, G.J., V. Mioulet, R.P. Kitching, M.E.J. Woolhouse, S. Andersen and A.I. Donaldson (2002). Foot-and-mouth disease virus infection of sheep, implications for diagnosis and control. Veterinary Record, 150: 724-727
Jamal SM and Belsham GJ (2013). Foot-and-mouth disease: past, present and future, Jamal and Belsham Veterinary Research, 44:116
Lefevere, C.P., J. Blancous, R. Chermette and G. UIlEngerg (2010). Infectious and parasitic disease of livestock. Laveisies. Paris, pp: 302-315
MacLauchlan, N.J. and E. Dubovi (2011). FENER’S Veterinary Virology. 4th ed. USA. Acadamic Press, pp: 32
Mekonen, H., D. Beyene, T. Rufael, A. Feyisa and F. Abunna (2011). Study on the prevalence of Foot and Mouth Disease in Borana and Guji Zones, Southern Ethiopia. Vet. world, 4: 293-296
OIE (2009). Foot-and-mouth disease. Chapter 2.1.5. Manual of diagnostic tests and vaccines for terrestrial animals. pp: 1-29
Paton, D.J., Di Nardo, A., Knowles, N.J., Wadsworth, J., Pituco, E.M., Cosivi, O., Rivera, A.M., Kassimi, L.B., Brocchi, E., de Clercq, K. and Carrillo, C. (2021). The history of foot-and-mouth disease virus serotype C: the first known extinct serotype? Virus Evolution, 7(1), p.veab009.
Press information bureau, Govt. of India, Ministry of Agriculture, 09- August-2016, FMD Mukt Bharat in next few years.
Radostits, O.M., D.C. Blood and C.C. Gay (2007). Veterinary Medicine, A Text Book of the Disease of Cattle, Sheep, Goats, Pigs and Horses. 8th ed. London: Balliere Tindall, pp: 1223-1227
Ranjan, R., Biswal, J.K., Subramaniam, S., Singh, K.P., Stenfeldt, C., Rodriguez, L.L., Pattnaik, B. and Arzt, J. (2016). Foot-and-mouth disease virus-associated abortion and vertical transmission following acute infection in cattle under natural conditions. PLoS One, 11(12), p.e0167163.
Rieder E, Berinstein A, Baxt B, Kang A and Mason PW (1996). Propagation of an attenuated virus by design: engineering a novel receptor for a noninfectious foot-and-mouth disease virus. Proc. Natl. Acad. Sci. USA. 93: 10428-10433
Rufael, T.C. (2006). Participatory appraisal and seroprevalence study of Foot and Mouth disease in Borana pastoral system, South Ethiopia. MSc thesis, Addis Ababa University, Faculty of veterinary medicine, Debrezeit, Ethiopia
Sammin D, Ryan E, Ferris NP, King DP, Zientara S, Haas B, Yadin H, Alexandersen S, Sumption K and Paton DJ (2010). Options for decentralized testing of suspected secondary outbreaks of foot-and-mouth disease. Transbound. Emerg. Dis. 57(4): 237-24
Upadhyaya, S., Ayelet, G., Paul, G., King, D.P., Paton, D.J. and Mahapatra, M. (2014). Genetic basis of antigenic variation in foot-and-mouth disease serotype A viruses from the Middle East. Vaccine, 32(5), pp.631-638.
Vegad JL and Katiyar AK. (2008). A textbook of veterinary special pathology (infectious diseases of livestock and poultry), international book distributing co, third reprint, 127
Venkataramanan, R., Hemadri, D., Bandyopadhyay, S.K., Taneja, V.K. (2006). Foot-and mouth disease in India: present status.Paper presented at a workshop on Global Roadmap for improving the tools to control foot-and-mouth disease in endemic settings. 29 Nov–1 Dec 2006, Agra
Verma AK, Kumar A, Mahima and Sahzad (2012a). Epidemiology and diagnosis of foot and mouth disease: a review. Ind. J. Anim. Sci. 82(6): 543-551

