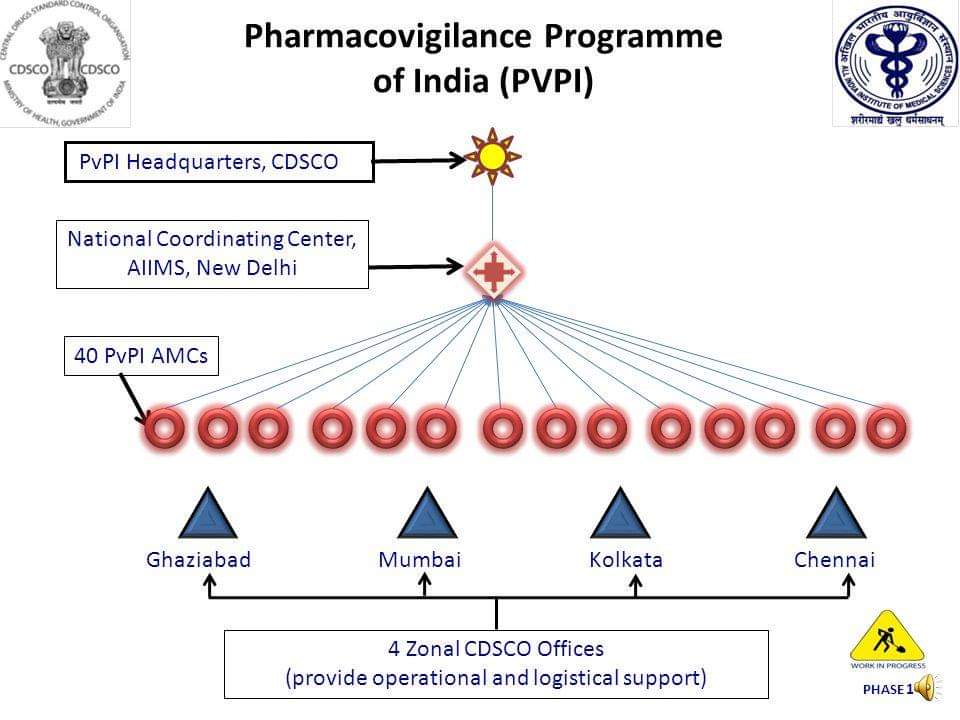
IMPORTANCE & NEED OF VETERINARY PHARMACOVIGILANCE IN INDIA
Veterinary pharmacovigilance (PV) is important for the Medicine which are used for treating disease in animals. It becomes more important when these animals are further used for producing food. Adverse drug reactions (ADRs) have a direct impact on animals and indirect impact on human beings, for example, through milk products, other animal producing food products. Currently, PV program of India is playing a vital role in assessing the safety of medicines in Indian Population. The safety of medicine in animals can be assessed by veterinary PV. The veterinary Pharmacovigilance is an important tool to ensure that a marketed drug is safe
Pharmacovigilance (PV) is a science relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem, came into existence to monitor the adverse drug reactions (ADRs) throughout the life period of a drug. In India, Ministry of Health and Family Welfare (MoHFW), Government of India (GOI) launched a nationwide PV Programme of India (PvPI) in the year 2010 to monitor the safety of drugs. Indian Pharmacopoeia Commission under the MoHFW functions as National Coordination Centre (NCC) for PvPI. NCC identified 202 ADRs monitoring centers across the country to monitor, identify and report ADRs to NCC.The mission of PvPI is “to safeguard the health of the Indian Population by ensuring that the benefits of the use of medicine outweigh the risks associated with their use.” The vision of PvPI is “to improve patient safety and welfare of Indian Population by monitoring drug safety and thereby reducing the risk associated with the use of medicines.” The medicines which are used for the treatment of animals shall be observed for their short- and long-term effects on animals and the effect on the environment because they may affect the ecosystem in one or other way. Unfortunately, there is an acute lack of information on veterinary PV in India. Drug regulatory authority of India banned the diclofenac sodium for animal use because of reducing number of vulture population and also regulate the use of injection oxytocin for animal use. Veterinary PV is same like PV, but it is related to use of medicines in animals. As per European Medicine Agency veterinary PV concerns “monitoring, evaluating, and improving the safety of veterinary medicines, with particular reference to adverse events in animals and human beings related to the use of these medicines.” It also involves the collection of information on adverse events due to off-label use and investigations of the validity of the withdrawal period and of potential environmental problems.
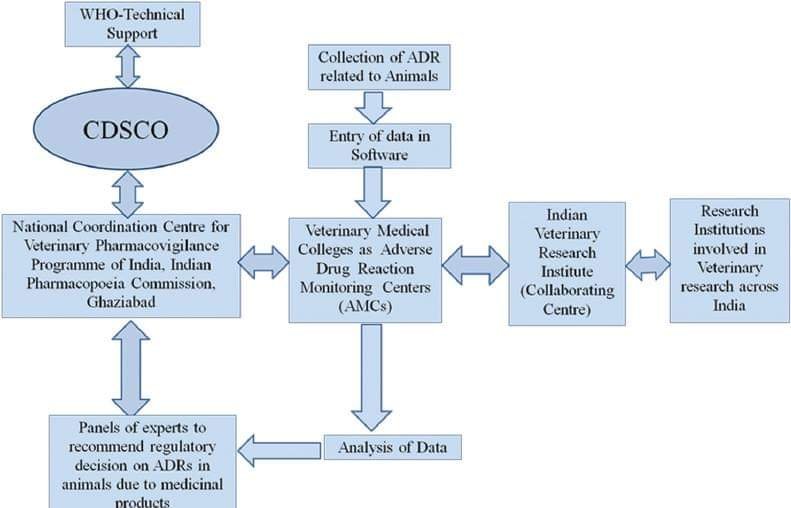
Recent studies reveal that ADR in animals is of major concern to the health of animals. ADRs have a direct impact on animals and indirect impact on human beings. The mortality rate of animals in India due to lack of veterinary PV is very high as compared to other developed countries. The concept of veterinary PV is relatively novel in India although many other countries such as USA, Canada, Europe, Japan, and China have well-established guidelines and systems for veterinary PV. Based on the recommendations of such systems few drugs have been banned by the respective regulatory authorities.
The UK veterinary medicine directorate states that the adverse events due to nonsteroidal anti-inflammatory drugs in various species of animals are similar. According to a study in France, eight signals were identified from the reports received or the periodic safety update reports. They resulted in revisions of the product information in sections addressing contraindications, adverse reactions, or withdrawal times.
The adverse events of medicinal products on animals need to be evaluated in line with international standards such as Veterinary International Conference on Harmonization of USA and European Union. There are many other countries who are viewing veterinary PV very seriously and are conducting research to understand the frequency of ADRs among various species of animals.
Veterinary medicines in India are regulated by Central Drugs Standard Control Organization. The Adverse Drug Events definition for animals is- “any side effect, injury, toxicity or sensitivity reaction (or failure to perform as expected) associated with the use of an animal drug, whether or not determined to be attributable to the drug (As per Centre for Veterinary Medicine, USFDA).
In India, there is a felt need to monitor the safety of drugs which are being used in animals. The consumption of dairy products and products obtained from animals are abundant in India. Hence, the medicines which are being consumed by animals may affect the human population and environment in many ways. Therefore, veterinary PV is important for the reporting of adverse drug events including serious adverse events following use of medicines in animals. It also helps in understanding the resistance of certain medicines in animals. The regulatory authority of India is very active to monitor the safety of medicines; the CDSCO issued a circular no- F. No. X-11026/64/2014-BD dated June 6, 2014, to all State/Union Territories Drugs Controllers regarding the use of antibiotics for treatment of food producing animals and in animal feed. A general awareness among the Indian population is lacking in case of veterinary pharmacovigilance, which needs to be augmented, however various measures being taken by Govt. of India to monitor the safety of medicines for animals. In India PvPI a robust system has been established with a capacity to collate, analyse and to identify drug safety signals. These evidence and scientific base information is provided to CDSCO for taking appropriate regulatory measures. It facilitates to establish a system for monitoring the safety of medicine used in veterinary health. There is strong need for a system for PV of veterinary medicine, just as the one that exists for medicines meant for use in humans. A system/mechanism for veterinary PV is proposed in . A lead role for various stakeholders such as animal research Institutes, Indian Veterinary Research Institute, Veterinary Hospitals, agriculture universities is envisaged for establishing veterinary PV for the welfare of animals and humanity.
Human and veterinary medicines in India are regulated by the CDSCO. For years, animal health experts and researchers in this medical field have been making a pitch for a separate regulatory agency and an effective pharmacovigilance (PV) programme for the sector, but their demand has fallen on deaf years. Though the regulators were forced to ban various veterinary drugs citing adverse effects on animals and environment, there is no fool-proof system in place to monitor vaccines and drugs. Recently, authorities have banned diclofenac sodium, used in the treatment of bacterial infections, for animal use because of reducing number of vulture population. The manufacture and distribution of oxytocin formulations and its active pharmaceutical ingredient (API) in the private sector was also banned last month citing its misuse by the dairy industry.
Safety of veterinary medicine is an ignored subject in India. We still fail to understand that the medicines being consumed by animals affect the human population and environment in various ways, as the consumption rate of dairy and other animal-derived products is very high here. The national drug regulator’s decision is indeed welcome, but we need to do a lot more to tackle the core issues in this sector.
As we know , monitoring veterinary medicines is a challenging and laborious task. The drugs have to be observed for their short- and long-term effects on animals and the impact on the environment. It becomes more important when these animals are further used for producing food. As a result, the need to monitor adverse reactions becomes all the more imperative. Institutes involved in animal research and veterinary hospitals can be considered as ADR monitoring centres to assess the safety of medicines.
Pharmacovigilance is a science which deals with relating to the adverse drug reaction, detection, assessment, understanding and prevention of adverse effects, particularly long-term and short-term adverse effects of medicines. Pharmacovigilance is an important and integral part of clinical research with a number of recent high-profile drug withdrawals, like Cerivastatin. Recently the pharmaceutical industry and regulatory agencies have raised the issue of Pharmacovigilance, because of withdrwal of a number of high profile drugs like Cervistatin.
Importance of Veterinary Pharmacovigilance-
A new medicine which is launched without long term safety
studies may not claim to be the therapeutically safe and
effective and may show harmful or life threatening effect.
Few decades ago in India, the safety evaluation of drug was
based on the chronic use of that drug. But this practice was
inaccurate and failed to claim complete safety. Considering
this fact, many Indian organizations or research funding
bodies started investing in individual drug research and
launching newer product (Huerta-Sanchez, 2015) .
Once product is developed a new information tends to be generated which may be positive or negative on risk-benefit profile of that product. Complete study or assessment of newly generated information with the help of Pharmacovigilance system is essential to safeguard the public health. The adverse effects of drugs could result in morbidity or mortality and study of which is essential to minimize risks and maximize benefits. Due to recent high-profile drug withdrawal, the pharmaceutical company and regulatory authorities are strict (Priyanka, 2014) .
Focusing on safety of drug in market i.e. Pharmacovigilance
India secured 4th rank in the global pharmaceutical
production. More than two different prescription or non-
prescription drugs at a time which may interact with each
other and produces discomfort. Hence, to avoid this situation
and protect the patients from potential harm caused by new or
existing drug there is need to improve the Pharmacovigilance
system. The Pharmacovigilance personnel keeps an eye on
adverse drug reaction (ADRs), analyses them accurately to
communicate results with stakeholders to ensure rational use
of drug (Gaies, 2012). It has been essential to meet the
challenges of the increasing range and potency of
pharmaceutical and biological medicines including vaccines,
which carry with them an inevitable and sometimes
unpredictable potential for harm.
Drug Information Centers and pharmacies throughout the country. It should also be made available to all primary healthcare centers (PHCs) in rural areas and all practicing general practitioners and physicians. Creating a clinical trial and post-marketing database. ADRs for signal detection and access to all relevant data from various stakeholders’ full complete data should be made available to the DCGI and to the various stakeholders from the date of first registration of the clinical trial in the India.
This data should comply with consolidated standards of reporting trials guidelines including overall benefit-risk profile of the product. Current standards of safety reporting as outlined in Schedule and information about all adverse events (AEs) and adverse drug effects (ADRs) per study arm should be systematically included as well as detailed description of cases with previously unknown adverse events (AEs) adverse drug effects (ADRs) and the reasons for study withdrawals, for drugs already in the market, type and frequency of all adverse events (serious and non-serious) should be submitted in periodic safety update reports (PSURs) and also added to the summary of product characteristics (SPCs).
List all new drug indications by maintaining a standard
database for every pharmaceutical company a list should be
maintained by the regulatory authorities and pharmaceutical
companies for all new drugs indications in the database. All
new issues need to be put under heightened surveillance.
Pharmaceutical companies in these circumstances should have
meetings set up with the DCGI to outline their risk
management plan (RMP) for the safety issues in question and
describe how they would put effective strategies in place to
mitigate the Education and training of medical students,
pharmacists and nurses in the area of pharmacovigilance
(Elhassan, 2015)
There are several courses conducted by various organizations
focusing in clinical research, but to date there is no course
relevant to pharmacovigilance in the country. The various
stakeholders including the MCI should incorporate a
pharmacovigilance syllabus within the pharmacology and
medicine curricula so that proper theoretical and practical
training can be imparted to physicians. Similarly, nurses and
Scope of Pharmacovigilance——–
The discipline of Pharmacovigilance (PV) has developed
considerably since the 1972 WHO technical report, and it
remains a dynamic clinical and scientific discipline. It has
been essential to meet the challenges of the increasing range
and potency of pharmaceutical and biological medicines
including vaccines, which carry with them an inevitable and
sometimes unpredictable potential for harm. The risk of harm,
however, is less when medicines are used by an informed
health profession and by patients who themselves understand
and share responsibility for their drugs. When adverse effects
and toxicity appear, particularly when previously unknown in
association with the medicine, it is essential that they are
analyzed and communicated effectively to an audience that
has the knowledge to interpret the information (Allabi and
Nwokirke, 2014) .
This is the role of Pharmacovigilance (PV), has already been
achieved, but more is required for the integration of the
discipline into clinical practice and public policy. To fulfill
the PV obligations for its marketed products as per
regulations, a pharmaceutical company in India has to
essentially carry out activities such as collection, and
expedited reporting of serious unexpected adverse drug effect
(ADRs). A typical setup for PV studies, including people
involved on various levels, organizational setup (Naik, 2015)
[24]. This is the role of Pharmacovigilance, of which much has
already been achieved. But more is required for the
integration of the discipline into clinical practice and public
policy. To fulfill the Pharmacovigilance obligations for its
marketed products as per regulations, a pharmaceutical
company in India has to essentially carry out activities such as
collection, and expedited reporting of serious unexpected
ADRs. A typical setup for Pharmacovigilance studies,
including people involved on various levels.
Current Scenario of Pharmacovigilance———
India is a vast country and there is a drug brand more than
6,000 licensed drug manufacturers and over 60,000 branded
formulations. India is the fourth largest producer of
pharmaceuticals in the world and is also emerging as a hub
for clinical trials. Many new drugs are being introduced in the
country, so there is an immense need to improve the
pharmacovigilance system to protect the Indian population
from potential harm that may be caused by some of the new
drugs (Yerramili, 2014) . In the past, India’s regulatory
agencies and drug companies based their safety assessments
on experiences derived from long-term drug use in the
Western markets and there was no real urgency for the
government to establish a strong pharmacovigilance system of
its own. In recent years, however, the lag between when a
drug is placed in the market and its subsequent availability in
India has decreased considerably so that the much needed
longer-term safety data is no longer available. In addition,
India-based drug companies have increased their capacity to
develop and launch new drugs through their own research
efforts and this has heightened the importance of developing
adequate internal pharmacovigilance standards to detect
adverse drug events (Mishra et al., 2013).
Inspections in all pharmaceutical companies operating in
India all pharmaceutical companies should be instructed to maintain and submit to the DCGI the Summary of
Pharmacovigilance System document operating within the
company, which would serve as the base for future
pharmacovigilance inspections. A high-level discussion with
various stakeholders, i.e., Ministry of Health and Family
Welfare (MHW), Indian Council of Medical Research
(ICMR), Medical Council of India (MCI), Pharmacy Council,
Nursing Council, Dental Council, Pharmaceutical Companies,
Consumer Associations, Nongovernmental Organizations
(NGOs) and Patient Groups should be initiated in order to
make them aware of how the drug control general of India
(DCGI) is planning to improve and develop a robust system in
pharmacovigilance Strengthen the DCGI office with trained
scientific and medical assessors for pharmacovigilance
Intensive training should be given in all aspects of
pharmacovigilance to officials working within the
pharmacovigilance department of the DCGI and in the
peripheral, regional and zonal centers. This should be an
ongoing activity with training scheduled twice a year.
Creating a single countrywide specific adverse event
reporting form to be used by all (Salim. 2015) A single countrywide specific adverse event reporting form
needs to be designed should not only be used by the National
Pharmacovigilance Centers, but also by all registered
hospitals (both private and government), teaching hospitals, pharmacists should also be trained in pharmacovigilance so that they are able to recognize adverse drug reaction (ADRs) and develop a culture of reporting ADRs in the future. An awareness program and a training schedule (both by distance education and face-to-face learning) covering all aspects of pharmacovigilance.
These are meant for the research and development (R and D)-
based pharmaceutical companies, particularly those involved
in new drug research, the medical profession, the pharmacists
and chemist-druggist trades and the patients, to be alert in
detecting ADRs and reporting them to the Indian regulatory
agencies, who in turn will investigate and take timely
corrective action. Collaborating with pharmacovigilance
organizations in enhancing drug safety with advancements in
information technology (IT), there has been the emergence of
new opportunities for national and internationals
collaborations that can enhance post-marketing surveillance
programs and increase drug safety. The Uppsala Monitoring
Center (UMC) is an example of an international collaboration
to establish a harmonized post-marketing surveillance
database. The system is based on the exchange of adverse
reaction information among national drug monitoring centers
in 80 countries. The information is transferred, stored and
retrieved in a timely and secure way through the internet
(Allabi and Nwokirke, 2014)
The UMC database collectively contains over four million
records with a large number of data fields. A similar database
can be built for the DCGI with the help of experienced private
firms from the safety data received from clinical trials and
post-marketing surveillance. Building a network of
pharmacovigilance and pharmacopeidemiologists in India
core group of experts will need to be formed which will have
representatives from multinational corporations (MNCs),
Indian pharmaceutical companies and personnel from the
regulatory authority (DCGI). Interaction with the IT sector in
building a robust pharmacovigilance system for India
Software programs developed can be used for collection and
analyses of data sets, determining trends of drug usage in
various disease areas, compliance, medication errors and drug
interactions leading to ADRs.
Future Prospects——-
As future prospects increase, PV systems capable to detect
new ADRs and taking regulatory actions are needed to protect
public health. Little emphasis has been put into generating
information that can assist a healthcare professional or a
patient in the decision-making process. The gathering and
communication of this information is an important goal of PV
Information about the safety of drug active surveillance is
necessary. When develop new methods for active post-
marketing surveillance, one has to keep in mind that the
important to collect complete and accurate data on every
Serious reported event. Spontaneous reporting is a useful tool
in generating signals, but the relatively low number of reports
received for a specific association makes it less useful in
identifying patient characteristics and risk factors PV methods
must also be able to describe which patients are at risk of
developing an adverse drug reaction (ADRs). As a source of
information, the PV approach would be consistent with the
growing patient involvement in drug safety (Flower, 2013)
The PV could play a role in identifying individual risk factors
for the occurrence of certain ADRs. In the future, PV has to
concentrate on the patients as a source of information in
addition to the more traditional groups, such as the health
professionals. At present, the DCGI should act quickly to
improve PV so as to integrate Good Pharmacovigilance
Practice (GPP) into the processes and procedures to help
ensure regulatory compliance and enhance clinical trial safety
and post marketing surveillance. An appropriately working
PV system is essential if medicines are to be used carefully. It
will benefit healthcare professionals, regulatory authorities,
pharmaceutical companies and the consumers. It helps
pharmaceutical companies to monitor their medicines for risk.
Post-marketing PV is currently a challenging and laborious
process, not only industry-wide, but also for regulatory
agencies (Ghewari, 2014) The aim of the PV is to receive the information, documentation of the work and knowledge online while giving priority to the new and important safety issues. Non-serious events have less priority than serious events but
important in comparing the changes in health, although they
are also screened routinely in present time, GlaxoSmithKline
has created a powerful new approach to Pharmacovigilance
(PV), integrating traditional, case-based PV methods with
disproportionality and data visualization tools. (Borja-
Oliveira, 2015) .These tools exist within a system
framework that facilitates in-stream review, tracking of safety
issues and knowledge management. This very innovative tool
and the processes will help to advance PV by improving
efficiency and providing new analytical capabilities. Similar
approach may be adopted by pharmaceutical companies for
prompt detection and analysis of ADRs. Transparency and
communication would strengthen consumer reporting, which
are positive steps towards involving consumers more in PV.
India is the fourth largest producer of pharmaceuticals in the world. Many new drugs are being introduced every year so every health care professional must have knowledge about the importance of ADR monitoring and pharmacovigilance. Government of India should make reporting of adverse reaction mandatory for veterinary colleges across the country. All veterinary colleges both private and public in the country need to monitor the effect of medicines given to their patients and report whether there is any adverse drug reaction. For improving veterinary pharmacovigilance in India our newly formed VCI should take up this matters to Govt. of India for its Formation & implementation.
Compiled & Shared by- Team, LITD (Livestock Institute of Training & Development)
Image-Courtesy-Google
Reference-On Request.