Methanogenesis and manipulation of ruminal fermentation by physical means
Dr Akash Rathod1* and Dr Atul Dhok1
1*Livestock Development Officer, Government of Maharashtra
1Assistant professor, Department of Animal Nutrition, Nagpur Veterinary College, Nagpur (MS) India
Abstract
Rumen is complex ecosystem of ruminant animal in which feed components first undergo fermentative digestion by microorganisms and then glandular digestion by the host. During fermentation the feed components i.e., carbohydrates, nitrogenous substances and lipids converted into volatile fatty acids (mainly acetic, propionic and butyric acids), carbon dioxide, methane and ammonia. Methane, a colorless, odorless gas, is produced predominantly in the rumen (87%) and to a small extent (13%) in the large intestines. Rumen methane is primarily emitted from the animal by eructation. In order to compare emissions of greenhouse gases GHG, the global warming potential (GWP) of the individual gases is used, with CO2 as the reference gas. The GWP of methane is known to be 21-fold greater than that of CO2 and the GWP of N2O is 310-times greater than that of CO2. However, since methane has no nutritional value to the animal, its production represents a loss of dietary energy to the animal. In general, methane production in cattle constitutes about 2–12% of dietary GEI (Johnson and Johnson 1995). Reduction in methane production can result from a decreased extent of fermentation in the rumen or from a shift in the VFA pattern towards more propionate and less acetate. Hence, several rumen manipulation strategies have been used to reduce methane emission. Physical manipulation is one of the methane reduction strategies which included in this article.
Keyword: Greenhouse gases, methane, rumen manipulation, physical means
Introduction
Ruminant animals have two metabolic compartmental systems that have different nutritional requirements: microbial metabolism in the rumen and mammalian metabolism in the tissues. Enhancing ruminant productivity involves meeting requirements in proper amounts and balance for both metabolic systems. In ruminants, feed components first undergo fermentative digestion by microorganisms and then glandular digestion by the host. Fermentative digestion is advantageous for substrates that cannot be digested by the host enzymes but is inefficient for digesting proteins, amino acids and sugars, because of losses in energy and nitrogen. Feed components (carbohydrates, nitrogenous substances and lipids) are degraded to various extents by the microbial population. The end-products of microbial fermentation are volatile fatty acids (VFAs, mainly acetic, propionic and butyric acids), carbon dioxide, methane and ammonia. The anaerobic condition of the ruminal ecosystem limits the availability of ATP, and, therefore, most of the substrate energy is retained in the end-products of fermentation. The conserved energy is used mainly in the biosynthesis of cellular constituents (carbohydrates, nitrogenous substances, lipids and nucleic acids), for maintenance functions of microbial cells, and for the increase of biomass, overall referred to as ‘microbial growth’. The amount of microbial mass formed was believed to be primarily dependent on the amount of energy conserved during substrate degradation (catabolism).
The ultimate aims in manipulating ruminal fermentation are to maximize the efficiency of feed utilization and increase ruminant productivity. In simple terms, the objectives of ruminal manipulation are to:
- enhance beneficial processes;
- minimize, alter or delete inefficient processes;
- minimize, alter or delete processes harmful to the host.
Manipulation of ruminal fermentation can be considered as an optimization procedure, whereby optimal conditions are sought by maximization and/or minimization of fermentation processes depending on the type and level of feeding and animal production. Examples of processes for which maximization is done are fibre degradation, lactate fermentation, and conversion of non-protein nitrogen to microbial cell protein. Processes that should be decreased include methane production, protein, peptide and amino acid fermentations, and absorption of ammonia. The ruminal fermentation is described as an integrated system consisting of an interactive network of reactions (Van Nevel and Demeyer, 1988).
Ruminal fermentation may be characterized quantitatively by the amount of organic matter (OM) fermented, the concentrations and relative proportions of fermentation products produced and the amount and efficiency of microbial protein synthesis. In the anaerobic ecosystem of the rumen, much of the energy in the fermented OM is retained in the products of fermentative processes (VFAs and microbial cells), with small losses of energy as methane gas and heat. The intermediate components of OM fermentation also serve as the monomers for microbial cell synthesis. Therefore, an inverse relationship exists between production of fermentation products (VFAs) and microbial cell synthesis (Leng, 1982). The predominant VFAs are acetate, propionate and butyrate, and the relative proportions are influenced by the diet. The manipulation of rumen fermentation can be done in following nutrients:
- Manipulation of carbohydrate fermentation
- Manipulation of nitrogen fermentation
- Manipulation of lipid fermentation
Manipulation of ruminal fermentation:
Ruminal microbial processes can be modified by intervention at three levels: dietary, animal and microbial. The first two approaches impact on ruminal fermentation indirectly by altering the feedstuffs or the physiology of the ruminant. Microbial intervention is more direct, whereby the fermentation pattern is altered through the action of certain compounds, micro or macrocomponents in the diet, on the microbes.
Manipulation of carbohydrate fermentation:
Rumen fermentation and VFA synthesis
Manipulation of carbohydrate manipulation
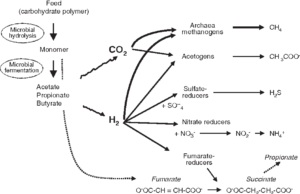
Rumen fermentation and VFA synthesis
I) Manipulation of carbohydrate fermentation
Cellulose and hemicellulose constitute 15-70% of most ruminant diets. They are insoluble, structurally complex, and not completely physically accessible, the extent of their fermentation in the rumen is very less. They also cannot be digested by the host enzymes. Increasing fiber or starch fermentation will result in increased VFA production in the rumen. As end-products of microbial metabolism, VFAs are the major sources of energy (up to 70%) for mammalian metabolism in the tissues, serving the role largely played by glucose in non-ruminants (Bergman, 1990).
So, the goal is to increase rate and extent of structural carbohydrate fermentation in the rumen to maximize nutrient intake and availability. This can be done by the following methods:
1) Processing of roughages:
The basic principle is that processing alters the physicochemical properties of the cellulose/hemicellulose contained in the feedstuffs, so they become more susceptible to microbial degradation in the rumen, increasing the propionate: acetate ratio in the rumen (Fahey et al., 1993).
2) Chopping of roughages: Smaller particles require less degradation in the rumen (Boadi et al, 2004).
3) Pelleting The voluntary consumption of pelleted forages is higher as the retention time is lesser, so efficiency of the animal increases with increase in propionate content. (Minson, 1963; Moore, 1964)
4) Ammonia/ urea treatment of roughages: It breaks the lignocellulosic bonds of the roughage; hence the fermentation process is easier. pH is also stable, and it favours the production of propionate over acetate.
5) Processing of grains: Many methods of processing grain have been also used to improve ruminal fermentation of starch, particularly of sorghum grain. (Orskov, 1979) Grinding and heat treatment processes are generally done to improve carbohydrate degradation and enhance the production of propionate over acetate, through the succinate pathway.
6) Level of feeding: With increased DMI over maintenance, the production of propionate is favoured. For animals with a high production level, forages must be supplemented with concentrates with a higher density of nutrients and less fibre. Due to less proportion of cell walls and more non-structural (readily fermentable carbohydrates like starch and sugar), concentrates favour propionic acid production, helping the animal to cater its energy needs for high productivity (Ilian et al., 1986).
7) Increasing proportion of concentrates:
Most energy-rich concentrates are associated with increased DMI, rate of rumen fermentation and feed-turnover rate, causing a greater change in the rumen environment and microbial composition. (Martin et al. 2010) However, high-concentrate diets are low in structural fibre and in the long term disturb rumen function by leading to sub-acute or acute acidosis; therefore, these diets are not sustainable for ruminant production. Feeding concentrate with a suitable Forage: Concentrate ration would obviously be effective in maintaining as animal productivity.
8) Increasing frequency of feeding
Increasing feeding frequency results in less variation in ruminal pH, which increase cellulolytic activity and microbial cell yield. (Nocek, 1992). Kaufmann et al. (1980) reported that when a given level of concentrate diets was fed to cows twice a day, rumen pH fluctuated from 5.85 to 6.65, whereas; when they were fed 6 times a day, rumen pH ranged only from 6.15 to 6.4.
9) Composition of concentrates:
Concentrates that are composed of different ingredients have variable carbohydrate compositions, ranging from structural (cellulose and hemicellulose) to non-structural (starch and sugar) carbohydrates. The degradable rate of both of these types of carbohydrates also varies widely according to the volatile fatty acid profile. Starch fermentation promotes propionate production in the rumen by creating an alternative H2 sink [Murphy et al., 1982], and a lower rumen pH [Kessel et al. 1996], decreasing the rumen protozoan numbers and limiting the interspecies H2 transfer between methanogens and protozoa [Finlay et al., 2002].
10) Replacing grass silage with maize:
Grass silage is usually harvested at a later stage of maturity, resulting in lower digestible organic matter, lower sugar and nitrogen contents and a fraction of lactate as a result of the ensiling process [Tamminga et al., 2007]. In contrast, maize silage or other whole-crop small-grain silage provides higher contents of dry matter with easily degradable carbohydrates, e.g., starch and sugar, increasing the DMI and animal performance [Beauchemin et al., 2007] There are three possible ways by which maize silage or whole-crop silage can manipulate carbohydrate fermentation in the rumen. First, the higher starch content favours propionate production rather than acetate. Second, the increased total DMI and passage rate reduce the ruminal residence time, thereby reducing ruminal fermentation and promoting post-ruminal digestion. Third, replacing grass silage with maize silage improves animal performance, by providing higher metabolizable energy [O’Mara et al., 1998].
II) Manipulation of nitrogen fermentation:
The logical strategy for manipulating nitrogen metabolism in the rumen is to enhance ruminal escape of dietary protein by minimizing its degradation and optimizing microbial protein production from NPN. Minimization of protein degradation can be achieved by intervening at the proteolysis, peptidolysis or amino acid deamination stages. This will reduce losses incurred in the conversion of dietary protein to microbial cell protein. Most efforts to improve urea utilization have been directed towards minimizing ammonia absorption by aiming to reduce the rate of urea hydrolysis in the rumen and/or to increase the ability of rumen microorganisms to assimilate ammonia, thus reducing nitrogen loss to the animal (Helmer and Bartley, 1971).
- The goal in manipulating microbial protein synthesis is to increase efficient production by improving ammonia assimilation and urea recycling, thereby minimizing nitrogen excretion. Urea recycling provides a great advantage when ruminants are fed low-protein diets
- This can be done by providing NPN sources to ruminants, so that the loss of N in the formation of microbial protein can be covered from urea, instead of the dietary protein.
- The microbial fermentation of soluble protein in the rumen is an unavoidable consequence of digestion and under many circumstances; it is a wasteful process because high quality proteins are broken down to ammonia, excess converted to urea in the liver and excreted through urine.
- The solubility of proteins change when subjected to special chemical treatment, which is advantageous to protect good quality proteins from rumen degradation.
- Number of chemicals like acetaldehyde, formaldehyde, glutaraldehyde, ethanol, tannic acid, acetic acid, sodium hydroxide etc. have been tried to protect proteins. Amongst all, aldehyde suggested for protection of protein, formaldehyde has been extensively used for production of bypass protein feed.
- Heating protein sources, so that they are undegradable at the rumen pH (6.5) is a common method to avoid loss to microbial protein synthesis. Usually, rumen degradability of crude protein from protein meals is 50-75%. As a result, net availability of amino acids for milk production is low. If these protein meals are given suitable chemical treatment to reduce rumen degradability of protein to 25-30 per cent, net availability of amino acids could be increased for milk synthesis. The amount of formaldehyde required to optimally protetct protein in different protein meals, without decreasing the digestibility of protein and essential amino acid is very important.
III) Manipulation of lipid fermentation
The acyl ester bonds of dietary lipids are hydrolyzed rapidly in the rumen by bacterial and protozoal lipases (Harfoot, 1978). The unesterified fatty acids are adsorbed onto the particulate matter, either feed particles or microbial cells (Harfoot et al., 1974), and are not degraded further because the anaerobic conditions in the rumen are unfavorable for oxidation of fatty acids. They may be incorporated into microbial lipids (Viviani, 1970). The unsaturated fatty acids are hydrogenated in the rumen primarily by bacteria and ciliated protozoa. Manipulation of ruminal lipid metabolism is aimed at two objectives
(1) Control of antimicrobial effects of fatty acids to minimize disruption of ruminal fermentation, so that higher levels of fat can be included in the diet; and
(2) Control of biohydrogenation to alter the absorption of selected fatty acids that may improve nutritional qualities of animal food products. Thus the goal in manipulation of ruminal fermentation is to minimize lipolysis. Efforts to increase flow of unsaturated fatty acids to the small intestine in ruminants have been stimulated by human health concerns about saturated fatty acids in animal products.
- The manipulation of biohydrogenation and loss of lipids to microbial lipid synthesis can be done by providing bypass fat to the animal.
- By pass fats can be prilled fats, or prepared by heating oils to a temperature that they get solidified and become undegradable at rumen pH.
- Calcium salts of fatty acids are also used as bypass fats as they are only disintegrated at acidic pH, i.e, the abomasum. Fats having calcium ions, instead of a glycerol backbone are inert in the rumen. Bypass fat has low solubility in the rumen and is less susceptible to biohydrogenation. However, in abomasum at acidic pH it dissociates and set free fatty acids and calcium for absorption. Feeding bypass fat to early lactating animals increases milk and fat yield and ensures early conception.
- Use of the bypass fat should be in the ration of dairy animals for 10 days before and 90 days after calving. It can be supplemented in the ration of dairy animals @ 15-20 g per kg milk production or 100 -150 g per animal per day. Feeding bypass fat does not hamper fibre digestion and is always beneficial than feeding ghee/oil.
- Advantages of bypass fat:
- Enhances peak milk production and persistency of lactation.
- Increase reproductive efficiency after calving
- Decreases metabolic disorders such as ketosis, acidosis & milk fever.
- Increases productivity and productive life of animals
Conclusion
Various physical strategies can be used to manipulate rumen function, increase the level and efficiency of animal performance, and minimize methane production and its adverse effects animal health and the environment.
References:
0rskov, E. R. (1979). Recent information on processing grain for ruminants. Livest. Prod. Sci., 6,335-47. 0rskov, E. R., Hine, R. S. and Grubb, D. A. (1978). The effect of urea on digestion and voluntary intake by sheep of diets supplemented with fat. Anim. Prod., 27, 241-5
Archimède H, Eugène M, Marie Magdeleine C, Boval M, Martin C, Morgavi DP, Lecomte P, Doreau M. Comparison of methane production between c3 and c4 grasses and legumes. Anim Feed Sci Technol. 2011;166-167:59–64.
Beauchemin KA, Kreuzer M, O’Mara F, McAllister TA. Nutritional management for enteric methane abatement: a review. Aust J Exp Agric. 2008;48:21–7.
Beever DE, Dhanoa MS, Losada HR, Evans RT, Cammell SB, France J. The effect of forage species and stage of harvest on the processes of digestion occurring in the rumen of cattle. Br J Nutr. 1986;56:439–54
Benchaar C, Pomar C, Chiquette J. Evaluation of dietary strategies to reduce methane production in ruminants: a modelling approach. Can J Anim Sci. 2001;81:563–74.
Boadi DA, Wittenberg KM, Scott SL, Burton D, Buckley K, Small JA, Ominski KH. Effect of low and high forage diet on enteric and manure pack greenhouse gas emissions from a feedlot. Can J Anim Sci. 2004;84:445–53.
Finlay BJ, Esteban G, Clarke KJ, Williams AG, Embley TM, Hirt RP. Some rumen ciliates have endosymbiotic methanogens. FEMS Microbiol Lett. 1994;117:157–61.
Kaufmann, W., Hagemeister, H. and Dirksen, G. 1980. Adaptation to changes in dietary composition, level and frequency of feeding. In: Y. Ruckebusch and P. Thivend (eds.). Digestive Physiology and Metabolism in Ruminants. h4″ Press, Lancaster, England. pp. 587-602.
Kessel JAS, Russell JB. The effect of ph on ruminal methanogenesis. FEMS Microbiol Ecol. 1996;20:205–10.
Madsen J, Lassen J, Hvelplund T, Weisbjerg MR. A fast, easy, reliable and cheap method to measure the methane production from ruminants, in Eaap publication no. 127. Wageningen: Wageningen Academic Publishers; 2010. p. 121 –2
Martin C, Morgavi DP, Doreau M. Methane mitigation in ruminants: from microbe to the farm scale. Animal. 2010;4:351–65
McAllister TA, Newbold CJ. Redirecting rumen fermentation to reduce methanogenesis. Aust J Exp Agric. 2008;48:7–13
Milich L. The role of methane in global warming: where might mitigation strategies be focused? Glob Environ Chang. 1999;9:179–201.
Minson, D. J. (1963). J. Br. Grassld SOC. 18, 39. Moore, L. A. (1964). J. Anim. Sci. 23, 230. Nicholson, J, W. G. & Sutton, J. D. (1969). B7.J. iVutr. 23, 585.
Murphy MR, Baldwin RL, Koong LJ. Estimation of stoichiometric parameters for rumen fermentation of roughage and concentrate diets. J Anim Sci. 1982;55:411–21. 37.
Nocek, J. E. (1992). Feeding sequence and strategy effects on ruminal environment and production performance in first lactation cows. I. Dairy Sci., 75, 3100-8.
O’Mara FP, Fitzgerald JJ, Murphy JJ, Rath M. The effect on milk production of replacing grass silage with maize silage in the diet of dairy cows. Livest Prod Sci. 1998;55:79–87.
Patra AK. Enteric methane mitigation technologies for ruminant livestock: a synthesis of current research and future directions. Environ Monit Assess. 2012;184:1929–52.
Tamminga S, Bannink A, Dijkstra J, Zom R. Feeding strategies to reduce methane loss in cattle. Lelystad: The Netherlands: Animal Nutrition and Animal Sciences Group, Wageningen UR; 2007, Report
Topps, J. H., Kay, R. N. B., Goodall, E. D., Whitelaw, F. G. & Reid, R. S. (1968). Br. J. Nutr.


