DR S.RAVI,MOET EXPERT, CHENNAI
MULTIPLE OVULATION EMBRYO TRANSFER (MOET) TECHNOLOGY IN DAIRY CATTLE : STEP BY STEP PROCEDURE
Cattle may be superovulated so that they will have multiple ovulations allowing numerous embryos to be collected on each flushing occasion. ET is also commonly referred to as MOET (Multiple Ovulation and Embryo Transfer), that refers to the whole process from superovulation of the donor female, flushing of the embryos, to the transfer of the embryos to the recipient female.Embryo transfer is a tool used by livestock breeders worldwide to assist in the transfer of genetics. Embryos may be frozen and shipped in liquid nitrogen that may be cheaper than physically transporting live animals.
Embryo transfer is a process by which an embryo is collected from a donor female and then transferred into a recipient female where the embryo completes its development. Embryo transfer is profitable for producers of registered purebred animals. Through the use of embryo transfer, a genetically superior female produces more offspring than she could by natural reproduction. The increased number of offspring thus maximizes the donor female’s genetic. Embryo transfer is used in several species of domestic animals, namely cows, horses, goats, and sheep. Research indicates its use in certain non-domestic species, such as deer, elk, bison, and wildcats. In the current period ET beyond doubt is being used in many developed and developing countries confined to certain research stations with success, however, still needs precautions in handling of embryos and evaluation of genetics progenies. Though this technique aims to improve the genetic progress of the herds and utilize the merit of the superior animals, nevertheless shadowed by the well documented adverse effects on the survival and wellbeing of the offspring.
In Vitro Fertilization (IVF) is also known as an Aspiration or Ovum Pick Up. During IVF, unfertilized eggs are harvested directly from the animal. While this process is typically used for cows and heifers, some dog breeders may also use the process.
Recovered eggs are fertilized one day after they’ve been aspirated. They’re then transferred roughly one week after they’ve been fertilized. This eight-day time period relies on an incubator for growth. The incubator itself has controlled temperatures, environments and media to mimic the host animal’s uterus.
Once the eggs have maintained the recipient’s standing heat for seven more days, they are transferred into the recipient animal. A lot of breeders who use In Vitro Fertilization have a unique opportunity: They can obtain more offspring from valuable females. IVF is particularly valuable to heifer and cow breeders as problematic females may otherwise have a lot of difficulty succeeding in regular breeding attempts.
Embryo Transfer
Embryo Transfer is a little more conventional than In Vitro Fertilization. It involves the use of specific hormones which have the follicle stimulating properties of donor animals. Like In Vitro Fertilization, embryo transfer is commonly used in large livestock animals—like heifers and cows.
During the embryo transfer process, donors are bred with artificial insemination. About seven days after artificial insemination, the embryos are “flushed” from the donor animal’s uterus. This process is non-surgical. The eggs are then either transferred to a new donor or are frozen to be used at a later date.
Breeders and livestock owners may opt for embryo transfer to increase an animal’s reproductive efficiency. In cows, embryo transfer can even increase year-round reproductive efficiency. This can give breeders the chance to multiply the success of superior pedigrees.
Which is Better?
In most cases, embryo transfer is a good choice for donors which produce high-quality embryos. Because the embryo cost during embryo transfer is low — and because it can result in many more offspring – it’s a good choice for breeders who want to breed many offspring from a reliable host.
In Vitro Fertilization, meanwhile, is more cost-effective when the donor produces fewer embryos. It’s also a better choice for breeders who want to keep their females stable in their regular production cycles. Cost-wise, In Vitro Fertilization may be more expensive up front. The actual cost of embryos, however, may be lower if the process is wisely invested in a healthy, high-value female.
BENEFITS OF EMBRYO TRANSFER FOR FARMERS.
Basically it multiplies the offspring of the farmers best animals. Farmers can use their best bulls over their best cow or heifer and get a good calf whereas now the farmer can run an embryo program and possibly get a life times’ production with one flush.
HORMONES.
There are three hormones that I know of and they are FSH, Follicle Stimulating Hormone, PG, Prostaglandin, and PMSG, Pregnant Mare Serum Gonadotropin. The FSH is taken from a cow’s Pituitaries, which is a gland in the brain that produces growth hormones and sex hormones. The PMSG is taken from a cows Serum, which is from the blood. PMSG and FSH cause the cow to super ovulate and the PG causes the cow to cycle.
PREPARATION.
First choose a donor cow for flushing, then insert a CIDR, which stands for Controlled Intra-vaginal Drug Release. The CIDR stops the cow from cycling by producing a hormone that makes the cow think that it is pregnant. Give the cow a series of injections morning and night with a drug such as Embryo S, which is a Follicle Stimulating Hormone that starts off at between 3.2 ml – 4.0 ml and works down each day to 0.8 ml. On the third day of injections also inject some Prostaglandin. Prostaglandin is a hormone causing the cow to cycle (come into season). After all the injections have been done and the CIDR has been pulled, watch the cow to find out the time when it comes on heat. Ten (10) hours after the cow has been on heat inseminate the donor cow with two straws of semen, then 12-18 hours later a second insemination using one straw of semen, then another 12 hours later a third insemination and finally 12 hours after that another insemination if you wish. Seven days later the donor cow is flushed and the embryos are isolated and then inserted in to the cows or frozen.
THE FLUSH.
After the preparations before the flush the cow is placed in a head bail then the cow is cleaned out, by getting all the poo out of the way then an instrument called an introducer is put into the cow’s vagina and the vet puts his hand up the cow’s bottom to help guide the introducer. It is pushed gently through the cervix into the uterus. The center piece is pulled out and a tube called a catheter is put in through the shell of the introducer and pushed right up into the uterine horn then a small balloon called a cuff at the end of the catheter is blown up. After the cuff has been blown up, a fluid for lubrication and nutrition of the embryos has to be injected. The fluid is a sodium chloride based phosphate that has a small amount of serum. The serum is an amber coloured liquid that separates from the clot when the blood hardens which is what we know as a scab. 210 – 300 mls of this fluid is injected through the tube and into the cows uterine horn, it is then sent back through the center tubing of the catheter in to an embryo filter. The extra fluid is released while the embryos are kept in the filter by a small grid on the bottom. After one side of the uterus has been flushed then the other side is flushed. When the flush is complete the embryo filter is washed out into a small container. The vets then search for embryos looking through a microscope. The embryos are graded and put into straws and implanted into the recipient cow or frozen for later use.
THE DIFFERENCE BETWEEN SURGICAL AND NON – SURGICAL TRANSFERS.
Besides the fact of trauma on the recipient the surgical transfer places the embryo further up the uterine horn than non – surgical. There may be a small increase in the conception rate maybe 5%, however if the recipients are in good shape it will not be of much benefit. Surgical transfer requires a small cut in the cows flank on the side of the ovulation. The uterus is pulled out and a small hole is made to the uterus. A small catheter is placed in through the hole and the embryo released. Non surgical is the same as A.I. A special gun is place up through the cow’s vagina and pushed through the cervix. The embryo is placed in the side of the ovulation and released from the gun.
CONCEPTION RATE.
With embryo transfer an already fertilised egg is being inserted, which eliminates one of the steps of artificial insemination so the conception rate should be 5 – 10% higher than artificial insemination, but a lot depends on the condition of the recipients. On an A.I. program the conception rate is 60 – 65% while on an embryo transfer program it is expected roughly the same sometimes a bit higher.
TEMPERATURES THAT EMBRYOS CAN BE STORED IN.
Fresh embryos can be stored at 37ºC for 6 – 8 hours without much harm but it is recommended that the embryos are inserted into the recipient cow or frozen as soon as possible. The embryos are frozen in liquid nitrogen, they can be stored in this for ever.
History of embryo transfer
Embryo transfer in cattle has recently gained considerable popularity with seed stock dairy and beef producers. Most of the applicable embryo transfer technology was developed in the 1970s and 1980s; however, the history of the concept goes back much farther. Embryo transfer was first performed and recorded by Walter Heape in 1890. He transferred two Angora rabbit embryos into a gestating Belgian doe. She went on to produce a mixed litter of Belgian and Angora bunnies. Embryo transfer in food animals began in the 1930s with sheep and goats, but it was not until the 1950s that successful embryo transfers were reported in cattle and pigs by Jim Rowson at Cambridge, England.
The first commercial embryo transfers in this country were done in the early 1970s. Initially, embryos were recovered from valuable donors and transferred to recipient animals using surgical procedures. It was not until non-surgical methods were developed in the late 1970s that embryo transfer grew in popularity.
| Events | Species | Scientist | Year |
| First successful ET | Rabbit | Walter Heape | 1890 |
| First successful ET | Rat | JS Nicholas | 1933 |
| First successful SOV | Cattle | Casida | 1940 |
| First successful ET | Sheep and Goat | BL Warmick & RO Berry | 1949 |
| First successful ET | Pig | AV Kvansnickii | 1951 |
| First ET reported in cattle | Umbaugh | 1949 | |
| First successful ET | Cattle | EL willett | 1951 |
| Baby girl born through ET | Steptoe & Edwards | 1979 | |
| Calf -Frozen thawed Embryo | Cattle | Wilmut & LEA Rowson | 1973 |
| Calf born by ET | Buffalo | Drost | 1983 |
| Calf born by IVF | Buffalo | Madan et al | 1990 |
| Calves through surgical and non-surgical ET in Asia/India
Calf born by frozen thawed embryo |
Buffalo |
Misra et al Misra et al., |
1988 1991 |
Work done in India
India is the fastest growing country with changing trends in economy and as per information from different sources more than 60 percent people are earning directly or indirectly from agricultural and animal husbandry. The contribution of agricultural and animal husbandry in Indian national GDP is equal (Patel, 2011). In India, initial isolated attempts of ET in dairy animals were made at the National Dairy Research Institute (NDRI), Karnal, Central Institute for Research on Buffalo (CIRB), Hisar, Indian Veterinary Research Institute (IVRI), Bareilly, and State Agricultural Universities. Simultaneously, the National Dairy Development Board (NDDB), Anand, initiated a pilot project in 1986 to investigate the utility of the ET technology for buffalo improvement and its encouraging results lead to the launching in 1987 of llational science and technolagy praject on Cattle Herd Improvement for Increased Productivity Using Embryo Transfer Technology, implemented by the Department of Biotechnology (DBT), Ministry of Science and Technology. The NDDB was designated as the lead implementing agency of the project and NDRI, Karnal, National Institute of Immunlogy (NII), New Delhi, CIRB, and IVRI were the collaborating agencies. The NDDB established its main ET Laboratory at Sabarmati Ashram Gaushala (SAG), near Ahmadabad and 4 regional laboratories at Animal Breeding Centre, Salon (UP), Shree Nasik Panchwati Panjarapole, Nasik (Maharashtra), Buffalo Breeding Centre, Nekarikallu (Andhra Pradesh) and Central Frozen Sernen Production Station and Training Institute, Hesserghatta(Karnataka). The NDDB also established 14 State ET centers at Bangalore, Bhubaneswar, Ernakulam, Erode, Jaipur, Caira, Kolhapur, Lalkuan, Ludhiana, Mehsana, Ongole, Pondicherry, Trivandrum and Ujjain. In the project, NDDB was assigned the role of creation of infrastructure of labs, skilled manpower and the pool of superior germplasm in terms of live calves and frozen embryos whereas collaborating agencies were primarily entrusted with the research in the areas related to folliculogenesis, superovulation, in vito fertilization, etnbryo sexing, embryo cloning etc. Subsequently, between 1992- 96, DBT supported the NDDB project on Embryo Transfer- Downstream Activities and Embryo Transfer-Upstream Activities of NDR1, NII and IVRI. DBT also supported the NDDB project on “Open Nucleus Breeding System” in Cattle Using Multiple Ovulation and Embryo Transfer (Misra et al., 2005).In India, due to ban on cow slaughter, almost all the embryos are produced either in vivo following superovulation and non- surgical embryo collection or in vitro following ultrasound guided trans-vaginal aspiration of oocytes (ovum pick-up) and subsequent in vitro, maturation (IVM). fertilization (IVF) and culture (IVC). In buffalo, however, most of the embryos are produced in vitro from the abattoir derived oocytes.
Applications of Embryo Transfer
1. Genetic Improvement
Embryo transfer is now commonly used to produce AI sires from proven donor cows and bulls in AI service. Although economics would not at this time support the use of embryo transfer techniques for anything but seed stock production, the commercial cattle industry can benefit by the use of bulls produced through well-designed Multiple Ovulation embryo Transfer (MOET) programs (Lohuis,1995).
2. Planned Mating
By far the most common use of embryo transfer in animal production programs is the proliferation of so-called desirable phenotypes. Embryo transfer provides the opportunity to disseminate the genetics of proven, elite females. Embryo transfer also permits the development of herds of genetically valuable females, most of which may be sibs if not full-sibs. Embryo transfer has also been used to rapidly expand a limited gene pool. As AI has led to the very valuable bull, embryo transfer has resulted in the very valuable female (Betteridge, 1981).
3. Genetic Testing
With this approach, it is possible to genetically test a bull in 3.5 years as opposed to 5.5 years using traditional progeny testing. Although accuracy may have been sacrificed, the shorter generation intervals can result in greater overall genetic gains.
4. Disease Control
Risk of infectios disease transmition is less by in vivo-produced embryos, providing embryo handling procedures were done correctly (Stringfellow et al., 2004). Several large studies have now shown that the zona- intact, washed, bovine embryo will not transmit infectious diseases. Consequently, it has been suggested that embryo transfer may be used to salvage genetics in the face of a disease outbreak.
Criteria for donor selection
There are two broad criteria for selecting donors for most embryo transfer programs:
(1) genetic superiorityand (2) likelihood of producing large numbers of usable embryos. In the majority of embryo transfer programs, in both developed and less-developed countries, superiority is determined in practice by market forces. In some cases, the sole criterion for selection is scarcity, and embryo transfer is used to increase numbers of animals available. This may be required to determine whether a new type of animal fits the environment or to get enough animals to develop appropriate management systems. If the objective is to conserve germplasm of indigenous breeds by cryopreservation of embryos, one may wish to select a random sample of donors (and sires) or insure that a range of phenotypes within the breed is used.
Selection of Sires
Since half of the genes come from the male, it is extremely important to use genetically superior bulls.In fact, selecting the male is usually more important than selecting the donor female because males will normally be bred to many females and can be selected more accurately than females. Likewise, it is necessary to select fertile bulls and fertile semen.
General procedural steps
The donor may be inseminated naturally or artificially and embryos will be collected non-surgically six to eight days after breeding. Following collection, embryos must be identified, evaluated and maintained in a suitable medium prior to transfer. At this point, they may also be subjected to manipulations, such as splitting and sexing, if necessary and may be cooled or frozen for longer periods of storage. Hence, the procedural steps must begin with the discussion of donor superovulation, insemination and then embryo transfer.
1. Superovulation
“Superovulation” refers to the release of many oocytes (eggs) during a single estrus period (Mapletoft et al., 2002). The objective of superovulation treatments is to obtain the maximum number of fertilized and transferable embryos with a high probability of producing pregnancies (Nogueira et al., 2002). Superovulation is a very inefficient method of obtaining oocytes from ovaries and is likely to be replaced by other approaches within the next decade. However, superovulation results in about ten times more embryos than single ovum recovery. Without superovulation, a usable embryo can be recovered about 60 percent of the time from normal donors by skilled technicians. There are various important factors associated with the success of this technique. These factors may include nutritional status, reproductive history, age, season, breed, ovarian status at the time of treatment and the effects of repeated superovulation.

The two generally accepted methods of superovulating cattle are based on two different gonadotrophins, although there are many minor variations of these methods. The simplest is to give an intramuscular injection of 1800-3 000IU (usually 2000-2 500IU) of pregnant mare’s serum gonadotrophin (PMSG), more correctly designated equine chorionic gonadotrophin (eCG), followed by a luteolytic dose of prostaglandin F2 alpha or an analogue intramuscular injection two to three days later (Pursley et al., 1995).

The second method of superovulation is to give eight to ten injections of follicle stimulating hormone (FSH) subcutaneously or intramuscularly. At half-day intervals, intramuscular injection is more reliable under field conditions. As with PMSG, prostaglandin F2 alpha is given 48-72 hours after initiation of treatment with the fifth, sixth, or seventh FSH injection. The most common FSH regimen is 6,6,4,4,2,2,2 and 2 mg at half-day intervals with prostaglandin F2 alpha given with the sixth or seventh FSH injection. About 20 percent more gonadotrophin should be given to cows weighing over 800 kg. Sometimes, higher doses are used for the first two days; others give 5 mg for each injection. There are few studies with adequate numbers of donors per treatment group in which constant and decreasing doses have been compared, so reliable conclusions cannot be drawn regarding efficacy of such regimens (Baruselli, 2006).
1. Insemination
Because of the release of many ova from multiple follicles, there is a greater need for viable sperm cells to reach the oviducts of the superovulated females. Therefore, many embryo transfer technicians will choose to inseminate the cow several times during and after estrus. One scheme is to inseminate the superovulated cow at 12, 24 and 36 hours after the onset of standing estrus. Using high quality semen with a high percentage of normal, motile cells is a very critical step in any embryo transfer program. The correct site for semen placement is in the body of the uterus. This is a small target (1/2 to 1 inch) just in front of the cervix.
2. Recovery of embryos
- Non-surgical recovery of embryos
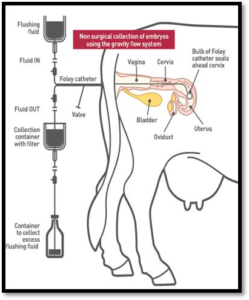
The first step in non-surgical recovery is to palpate the ovaries per rectum to estimate the number of corpora lutea. Ultrasonography provides more accurate information about responses than palpation (Kunkel, 2002). Epidural anaesthesia is recommended for non-surgical recovery procedures. The tailhead should be clipped, then scrubbed with iodine soap and swabbed with 70 percent alcohol to prevent infection of the spinal column. It is recommended to inject 5 ml of a sterile 2 percent solution of procaine in water using a new 18-gauge needle each time. Recovery procedures are carried out by manipulation per rectum. To collect the embryos non surgically, a small synthetic rubber catheter (Foley catheter) is inserted through the cervix of the donor cow, and a special medium is flushed into and out of the uterus to collect the embryos seven days after estrus (Hasler, 2004).
 |
|
Two-way Foley catheters with and without balloon inflated
Non-Surgical recovery of embryos is relatively simple and can be completed in less than an hour without harm to the cow. Apresterilized stylet is placed in the lumen of the catheter to offer rigidity for passage through the cervix into the body of the uterus. When the tip of the catheter is in the body of the uterus, the cuff is slowly filled with approximately 2 ml of normal saline. The catheter is then gently pulled so that the cuff is seated into the internal orifice of the cervix. Additional saline is then added to the cuff to completely seal the internal orifice of the cervix. AY connector with inflow and outflow tubes is attached to the catheter. A pair of forceps is attached to each tube to regulate the flow of flushing fluid. The fluid is sequentially added and removed by gravity. The fluid in the uterus is agitated rectally, especially in the upper one third of the uterine horn. The uterus is finally filled with medium to about the size of a 40day pregnancy. One liter of fluid is used per donor. Each uterine horn is filled and emptied five to ten times with 30 to 200 ml of fluid each time, according to size of the uterus. The embryos are flushed out with this fluid and collected in a filter with the fluid. The pores in the filter are smaller than the embryos, so excess fluid drains out of the filter without losing the embryos. Embryos are separated from the flush media and examined under a microscope to determine their quality and stage of development (George et al., 2008).
i. Surgical recovery of embryos
The first successful cattle ET studies obtained from the embryos by a surgical procedure. The donor, which had fasted and been tranquilized, was anaesthetized by intravenous knockdown injection with a halothane/oxygen mixture. This method of recovery is done by performing a Laparotomy (flank or midline abdominal incision) to expose the reproductive tract. A clamp or the thumb and forefinger can be used to block the distal one-third of the uterine horn, so that fluid injected into that segment can be forced through the oviduct with a gentle milking action and collected at the infundibulum. An alternate procedure is to occlude the uterine horn at the body of the uterus. Culture medium is introduced through a puncture at the uterotubal junction or through the oviduct until the uterus is turgid. The uterus is then punctured with a blunt needle attached to a flexible catheter. The pressure will cause the medium to gush through the catheter, with enough turbulence to carry the embryos into a collection tube. These procedures allow for the recovery of a high percentage of embryos. However, because of the surgical trauma and resulting adhesions they can be repeated only a few times (Peregrino, 2000). Surgical recovery can be done in all species and is the method of choice for sheep, goats, and hogs. Techniques vary slightly with the species (Betteridge, 2003).
1. Embryo handling, evaluation and storage
- Embryo Handling
 |
|
Careful handling of embryos between collection and transfer is necessary to prevent the transmission of pathogens (Peregrino, 2000). Embryos are located under 10 X magnification with a stereoscopic dissecting microscope after filtering the collection medium through a filter with pores that are approximately 50- 70 μm in diameter. Embryos are normally held in the same or a similar medium to that in which they were collected. Media must be buffered to maintain a pH of 7.2 to 7.6 and have an osmolarity around 300 molar osmolarity. Dulbecco’s PBS or more complex media with the Hepes buffer and enriched with FCS and antibiotics are normally used in the field. More complex
media with a carbonate buffer generally yield superior results for long term culture of bovine embryos. As indicated earlier, embryo collection holding and freezing media that are free of animal products have recently become available, avoiding the need for refrigeration and increasing biosecurity (IVIS, 2002).
ii. Embryo Evaluation
Embryos are classified and evaluated by morphological examination at 50 to 100 X magnification. The overall diameter of the bovine embryo is 150 to 190 µm, including a zona pellucida thickness of 12 to 15 µm. the quality of an embryo is evaluated and graded as excellent, good, fair and poor depending on several factors discussed below (Dochi et al., 1998):
Quality evaluation
Excellent: An ideal embryo, spherical, symmetrical and with cells of uniform size, color and texture.
Good: Small imperfections such as a few extruded blastomeres, irregular shape and a few vesicles.
Fair: Problems that are more definite are seen, including presence of extruded blastomeres, vesiculation, and a few degenerated cells
Poor: Severe problems, numerous extruded blastomeres, degenerated cells, cells of varying sizes, large and numerous vesicles but an apparently viable embryo mass. These are generally not of transferable quality (Mapletoft et al., 2002).
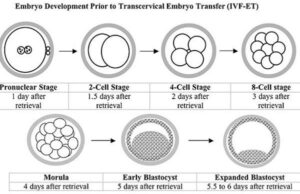
Embryo Developmental Stage Evaluation
Embryos also are evaluated for their stage of development without regard to quality. It is advisable to select the stage of embryo development for the synchrony of the recipient (Stroud, 2012):
 |
|
i. Embryo storage
Donor embryos can be transferred immediately into recipients, or they can be stored for future use, hence it may be
necessary to store embryos until suitable recipients become available for transfer (Sauve, 2002).
a. Short-term storage
Embryos can be stored at room temperature for one day for direct transfer from the donor to the recipients. For periods of 24 to 72 hours, the embryos must be stored at 4°C in PBS, medium 199, or medium L15, each supplemented with 50% FBS. Most media and culture systems are adequate for maintaining the viability of the embryo between donor and recipient (Atsushi et al., 2013).
b. Long-term storage
If embryos are to be transported great distances or suitable recipients are not immediately available, a long-term storage system is essential. Deep-freezing embryos is storage in liquid nitrogen (-196°C) for an indefinite period of time. Long-term storage through freezing usually results in damage of 30% to 50% of the stored embryos. Damage is usually caused by ice crystal formation within the embryonic cell. Although the average survival rate of frozen-thawed embryos is approximately 65%, it is profitable to maintain embryos in long-term storage (Atsushi et al., 2013).
1. Selection of recipient, transfer of embryo and post transfer management
- Selection of recipient females
Proper recipient herd management is critical to embryo transfer success. Cows that are reproductively sound, that exhibit calving ease and that have good milking and mothering ability are recipient prospects. They must be on a proper plane of nutrition (body condition score 6 for beef cows and dairy body condition score 3 to 4). These cows also must be on a sound herd health program.
ii. Synchronization of the recipients
To maximize embryo survival in the recipient female following transfer, conditions in the recipient reproductive tract should closely resemble those in the donor. This requires synchronization of the estrous cycles between the donor and the recipients, optimally within one day of each other. Synchronization of the recipients can be done in a similar manner and at the same working time as the donor cows (Galina and Orihuela, 2007).
2. Transfer of bovine embryo
- Surgical Transfer
Embryos can be transferred via mid-line abdominal incision to cows under general anaesthesia, but through flank incision is far more practical. About 60 ml of 2 percent procaine is given along the line of the planned incision. A skin incision about 15 cm long, high on the flank is made just anterior to the hip. The ovary, is located usually about 25 cm posterior to the incision, and CL is visualized or palpated. The uterine horn is exteriorized by grasping. A puncture wound is made with a blunted needle through the wall of the cranial one- third of the exposed uterine horn, because it is very fragile. Using about 0.1 ml of medium in a small glass pipette (<1.5 mm outside diameter), embryo is drawn up from the storage container. The pipette is then inserted into the lumen of the uterus, and the embryo is expelled (Marahall and Minyard, 2002).
ii. Non-Surgical Transfer
First, it is necessary to palpate ovaries accurately in order to select the side of ovulation. The next step is to pass the embryo transfer device through the cervix. The petridish containing the embryo is placed under the stereoscope to load in a 0.25 ml transfer straw. The straw is then loaded into a transfer gun and covered with a transfer sheath. The third step with non- surgical transfer is to be able to insert the tip of the instrument into the desired uterine horn quickly, smoothly and atraumatically. Nonsurgical transfer is preferable, because it is less expensive, it is quicker and does not involve surgical procedures. The most commonly used instrument for non-surgical transfer is the standard Cassou Minseminating gun for French straws, because it is inexpensive and easy to use correctly.
Factors Affecting the Success of Embryo Transfer in Cattle
Many factors may influence the embryo transfer technology that may be beyond the control of practitioners.
- Long term weather problems or storms during the superovulation/recipient synchronization process are beyond the control of anyone and can wreak havoc with ET success (Armstrong and Evans, 1983).
- Travel problems sometimes means traveling to a farm a day or two late, which mandates working with older embryos than planned. Probably the single most important variable affecting success in ET is the level of donor and recipient management (Schneider al., 1980).
- The risk of transmitting genetic disease via embryo transfer is the same as that involved in natural mating or artificial insemination; wise selection of dams and sires is mandatory, (King et al., 1985).
Advantages of embryo transfer
- Increased reproductive potential: This should be considered in relation to the female The sperm that can be obtained from a genetically superior bull and be utilized widely through artificial insemination.
- Naturally, the genetically superior cow can produce approximately 10 calves in its lifespan but through adoption of ET, there is a notable increase in the number of offspring that a genetically superior cow can produce through surrogates.
- Relatively faster genetic improvement: There is a noticeable positive upward trend based on the different techniques adopted to improve a herd’s genetic For example, when using natural breeding, it could take up to 20 years while when using AI, the time span is reduced almost by half. The use of ET reduces this time further to approximately four to five years.
- Outsmarts natural catastrophes: At times we may have a very good and genetically superior cow that has suffered disease bouts, injuries or has grown relatively old and become This animal is often considered infertile but through embryo transfer technology, the animal can be super ovulated to harvest its ova and fertilise them in-vitro.
- Environmental advantage: In ET, passive immunity is passed on by the native dam, giving the offspring a better chance of survival despite the embryo being 100 per cent genetically different from the surrogate
- Financial benefits: Once the desired genetic impact is realised, the production of milk and beef will improve culminating to increased In well-established enterprises, one can also undertake selling of the embryos as additional income.
- Embryos can also be stored indefinitely through freezing, thus making the highly superior genetic material available for future use. This technology is also considered cheaper than exporting/importing live animals.
Disadvantages of Embryo Transfer
- Technical specificity: The technology requires trained technical personnel and a high level of It needs technical knowledge especially in relation to flushing the embryos and observation of oestrus in the recipient.
- High cost: The high cost can be attributed to the necessary equipment that must be put in place and the maintenance of recipient animals, among other However, governments can establish these stations where the service can be accessed when necessary.
- Reduced genetic variability: The relatively small gene pool sourced from the donor cows may have a negative impact in future especially in reference to the rare breeds.
- Low success rate: The success rate of embryo transfer is reportedly lower than use of artificial This may be attributed to the numerous processes that have to be undertaken before the embryo is successfully implanted.
- This being a labour intensive and time consuming technology, it may be prudent that farmers weigh their options and understand their abilities and goals before embracing embryo transfer technology.
Costs of embryo transfer
Costs of embryo transfer vary greatly from country to country, and within countries, depending on a variety of factors. No matter how it is done, embryo transfer programs are relatively expensive. The costs of the actual embryo transfer services or technology may be quite low; however, labour and feed costs averaged over the number of calves produced are high since normal, healthy cows or heifers are kept out of production in order to be available as recipients. Although embryo transfer is generally costly, it is frequently still profitable. Obviously, one must analyse the costs and benefits. When the benefits exceed the costs, this technology would be used by all means. However, if costs are not justified by benefits, embryo transfer programs should not be initiated in most cases.
In conclusion, commercial embryo transfer in cattle has become a well established industry throughout the world. Although a very small number of offspring are produced on an annual basis, its impact is large because of the quality of animals being produced. Embryo transfer is now being used for real genetic improvement, especially in the dairy industry, and most semen used today comes from bulls produced by embryo transfer. An even greater benefit to embryo transfer is that in vivo-produced bovine embryos can be made specified pathogen-free by washing procedures, making this an ideal procedure for disease control programs or in the international movement of animal genetics. Techniques have improved over the past 60 years so that frozen-thawed embryos can be transferred to suitable recipients as easily and simply as artificial insemination. In vitro embryo production and embryo and semen sexing are also successful, but time and cost limit their widespread use. A combination of embryo transfer using proven cows inseminated with semen from proven bulls, followed by industry-wide artificial insemination appears to be the most common use of bovine embryo transfer in the near future.
Step-by-step guide to carrying out IVF on cows
A growing number of cattle breeders are using in vitro fertilisation (IVF) to maximise pregnancy rates.
This is the process of harvesting oocytes from donor cows, and creating embryos by fertilising the oocytes with semen in a petri dish.
The embryo is then implanted into a recipient (otherwise known as surrogate cow), or they can be frozen indefinitely.
How the procedure works
The first stage is the key addition to conventional embryo transfer. Eggs are removed from the donor cow’s ovary before the ovary naturally releases the oocyte down the fallopian tube.
Using a process called trans-vaginal recovery, IVF works by first removing the dominant follicle in the ovary, allowing the rest to grow.
In a normal pregnancy, the dominant follicle inhibits the rest – otherwise a cow could end up developing dozens of calves in the womb, she adds. With IVF, all follicles are left for six days to develop uninhibited within the ovary in a process called dominant follicle regression (DFR).
Ooctye development
1 Dominant follicle regression
The donor cow is given a local anaesthetic and cleaned with a mild disinfectant and saline solution. This can happen on the farm or at the Paragon facility.
2 Ultrasound needle
An ultrasound-guided needle enters the ovary to remove the dominant follicle and stimulate super-ovulation.
3 Super-ovulation
a three-day course of follicle stimulating hormones (FSH) is administered to stimulate ovaries to produce more oocytes.
Oocyte collection
4 Ovum pick-up (OPU)
The donor cow is brought into the crush collection facility, where the temperature is at 27-32C. Oocytes are harvested via trans-vaginal recovery, averaging 10 eggs per collection, and the eggs are matured for 20 hours.
5 Insulated chambers
Eggs are placed into insulated chambers at 37C to mimic a cow’s body temperature.
Fertilisation
6 Fertilisation occurs with semen
7 Embryos
Resulting embryos are matured for a week in the laboratory, passing through eight different maturation liquids (medias) mimicking the changing pH and gas levels inside the uterus.
8 Embryos can be transferred directly or frozen indefinitely
Recipient and donor management advice
General guidelines Recipient cow or heifer · Avoid lush, wet grass – if grazing, buffer-feed if possible. · Indoor recipients achieve 5-10% better pregnancy rates that those managed at grass.
· Bought-in stock should have six weeks to settle into new farm.
· Recipients should be managed as a group and major changes (such as spring turn-out and autumn housing) should be avoided for six weeks pre- and-post transfer.
· Good body condition score is essential – 2.5 minimum.
· A long fibre-based diet of hay, big bale silage or straw should be fed.
· Low-protein coarse mix as a supplement is preferable (although not barley).
· Sugar beet pulp can deliver energy and fibre – and be used as a mineral carrier.
· Routine treatments (worming, vaccination, foot trimming) should be avoided at this time.
Cows · Cows calve easier and so the process can be preferred in beef breeding or with low calving ease bulls.
· Works best on fourth-calvers or younger.
· Select cows with no reproductive or health issues.
· Synchronise cow after peak yield as stress of lactation can reduce pregnancy rates.
· Pregnant cows can be collected from until about the fourth month of pregnancy.
Maiden heifers
· Heifers average 5-10% better pregnancy rate, compared with cows.
· Should be at least 15 months old, cycling regularly and weigh 350kg (depending on the breed)
IVF stages – fortnight timelines
Donor cow Laboratory Recipient cow Day 1 Day 2 to 6 Day 7 Day 7/8 Day 8 Day 8 to 15 Day 15 DFR Superovulation after three-day FSH course OPU Egg maturation 20-24 hours Fertilisation Maturation in incubator Must be used within 24 hours if fresh in cow seven days after heat Benefits of using IVF
- 50% of usual amount of semen needed. Half a straw can inseminate around six or seven donors
- About 10 eggs per collection. About 73% of these will be fertilised
- Then 43% will become a viable and freezable
- Fresh pregnancy rate = 58%
- Frozen pregnancy rate = 57%
Breeding benefits
Problem breeders can be salvaged because they can be used as a donor if, for example, they have:
- Uterine damage
- Fallopian tubes blocked
- Scarring
Extend breeding life:
- Collection possible from 10 months to 20 years old and above
- Ovaries can be recovered from abattoir
- Condition:Heifers must be well-fleshed but not too fat. Too much weight is an “uphill struggle” and should mean heifers are ruled out of embryo transfer work. With reference to fatness classifications on the Europ grid, Mr Savage targets 3s.
- Nutrition:Energy levels must be kept high, with no sudden changes in diet in the two months leading up to oocyte collection and embryo transfer.
- Regime:Quality semen must be used, and optimum care taken of semen and embryos to maximise conception.
IVF contenders must be mature cows with calves on the ground of exceptionally high genetic merit that may be experiencing breeding difficulties.
Donors: five weeks out
- Flax oil: 50-60ml daily
- Dried sugar beet: 1-1.5kg a head a day
- High-energy/low-protein pre-calving nuts (40-50% maize, 14%CP): 1-1.5kg a head a day
- Pre-calving minerals. Mixed with sugar beet and pellets or placed on to silage
Recipients: six to seven weeks after transfer
- Broadly the same protocol
- One difference is that double the amount of flax oil is used (100ml a head a day)
- Cows are scanned at seven weeks and then transitioned on to standard heifer diet if pregnant.
PDF OF BOOK ON EMBRYO TRANSFER TECHNOLOGY (ETT) CAN BE DOWNLOADED FROM HERE:Cattle Embryo Transfer Procedure
References:ON REQUEST
PHOTO-CORTTESY-GOOGLE
- https://www.pashudhanpraharee.com/embryo-transfer-technology-ett-in-dairy-cattle/
- https://www.dvs.gov.my/dvs/resources/user_16/MJVR%20Vol9%20No%202/MJVR-V9N2-p109-116.pdf


