Navigating the Complexities of Bird Flu: From Etiology to Global Control
Deshmukh Kaivalya Ruprao1, Hemanth kumar2, Ashmita debnath2, G Yashaswini2 , Ambika Nayak3, Mate Devyani3
1M.V.Sc Scholar, Division of Animal Biochemistry, Indian Veterinary Research Institute, Izatanagar, Bareilly – 243122. Email ID – kaivalya.deshmukh17@gmail.com
2PhD Scholar, Division of Animal Biochemistry, Indian Veterinary Research Institute, Izatanagar, Bareilly – 243122. Email ID – kumarhemanth360@gmail.com
2PhD Scholar, Division of Animal Biochemistry, Indian Veterinary Research Institute, Izatanagar, Bareilly – 243122. Email ID – drashmitadn11@gmail.com
2M.V.Sc Scholar, Division of Animal Biochemistry, Indian Veterinary Research Institute, Izatanagar, Bareilly – 243122. Email ID – yashuramya92@gmail.com
3PhD Scholar, Division of Veterinary Microbiology, Indian Veterinary Research Institute, Izatanagar, Bareilly – 243122. Email ID – ambikanayak399@gmail.com
3PhD Scholar, Division of Veterinary Microbiology, Indian Veterinary Research Institute, Izatanagar, Bareilly – 243122. Email ID – devyanimate@gmail.com
Abstract:
Bird flu, caused by the Highly Pathogenic Avian Influenza (HPAI) H5N1 subtype, is a major threat to birds and humans. With economic impacts and zoonotic concerns, outbreaks primarily affect domestic poultry, originating from wild bird reservoirs. Significant antigenic changes may lead to pandemics, with pigs serving as potential mixing hosts. The potential for human-to-human transmission raises the risk of a devastating pandemic. Molecular techniques aid diagnosis, and WHO guidelines are vital for preparedness. Novel approaches include vaccines and universal antibody-based therapies. Inactivated and recombinant vaccines are globally used, while Tamiflu is a common medication. Herbal medicine is gaining attention for its potential in managing human illness.
Keywords: Bird flu, avian flu, influenza virus, HPAI,H5N1,Poultry, zoonosis, Prevention ,Diagnosis, Control and Treatment
- Introduction:
Bird flu, also known as avian influenza or fowl plague, is a highly threatening viral disease affecting domesticated birds, often causing up to 100% flock mortality. This economically significant illness possesses zoonotic potential and poses potential pandemic threats. The Avian Influenza Virus (AIV) is the causative agent, ranging from subclinical to highly virulent infections in poultry. First detected in Italian poultry flocks in 1878, the virus has since led to global outbreaks. Free-flying migratory and wild birds, especially ducks and geese, serve as important AIV reservoirs with mostly asymptomatic infections. The disease is classified as Highly Pathogenic Avian Influenza (HPAI) or Low Pathogenicity Avian Influenza (LPAI) based on pathogenicity.HPAI is a highly contagious, multi-organ systemic disease listed by the World Organization for Animal Health (OIE). Control is challenging due to severe genetic alterations, and the virus has evolved to affect not only poultry but also migratory birds, mammals, and even humans.
|
|
||||
2. Etiology:
Avian or bird flu is caused by the Avian Influenza Virus (AIV), a Type A influenza virus belonging to the Orthomyxoviridae family. AIV is a negative-sense single-stranded RNA virus with a diameter ranging from 30 to 120 nm. It is an enveloped virus featuring “spikes” or “projections” measuring 10-12 nm in length, comprising rod-shaped Hemagglutinin (HA) and mushroom-shaped Neuraminidase (NA) glycoproteins. Influenza viruses are categorized into subtypes based on haemagglutinin (H1-17) and neuraminidase (N1-10) antigens. Birds have been found to harbor all 16 H and 9 N subtypes, and more recently, H10 and N10 subtypes have been identified. Highly fatal infections are associated with the H5 and H7 subtypes, characterized by multiple basic amino acids at the cleavage site of the HA protein. The HA glycoprotein is responsible for hemagglutinating activity, attaching the virus to host cells and eliciting protective antibodies post-infection. Meanwhile, the NA glycoprotein facilitates the release and spread of new virus particles from the infected cell. The virus is sensitive to environmental factors such as heat, pH levels, and dryness, making it susceptible to various detergents and disinfectants. Long-term storage of the virus is recommended at -70°C following lyophilization. Avian influenza virus can be inactivated at temperatures of 56°C for 3 hours, 60°C for 30 minutes, 80°C for 3 minutes, and 80°C for 1 minute.
- Host Range, Transmission, and Spread:
The avian flu virus naturally infects a diverse range of bird species, primarily affecting domesticated poultry like chickens and turkeys. Turkeys and chickens are particularly susceptible, with other species such as quails, guinea fowls, pheasants, partridges, geese, ducks, ostriches, passerine birds, and pheasants also at risk.
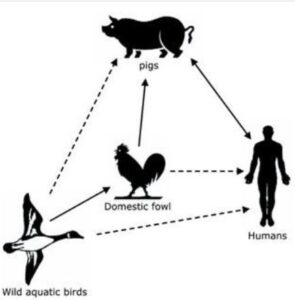
Fig:Transmission cycle of Bird flu:
This highly contagious virus can quickly spread globally, often entering a country through wild fauna and then infecting domestic flocks through direct or indirect contact. Transmission occurs via direct contact, airborne droplets, or exposure to contaminated surfaces, including feed, equipment, and clothing. Feco-oral transmission is a major route, with a small amount of infected bird feces having the potential to infect a large number of birds. Wild and migratory birds, especially ducks and geese, act as significant reservoirs by continually shedding the virus through respiratory secretions and droppings. Various subtypes, including H5N1, H5N2, H5N3, H5N8, H7N7, H9N2, H10N7, H7N9, H1N1, H2N2, H6N1, and H3N2, have been reported in poultry.
- Role of Migrating Birds in Spreading Bird Flu:
4.1. Virus Shedding: Migrating birds, especially ducks and geese, carry avian influenza virus (AIV) without exhibiting symptoms, shedding the virus in respiratory secretions, feces, and saliva.
4.2. Long-Distance Migration: The vast distances covered by migrating birds facilitate the global spread of AIV, introducing the virus to new regions.
4.3. Contamination of Water Bodies: Birds contaminate water sources along migration routes, contributing to the spread of AIV as other birds come into contact with contaminated water.
4.4. Contact with Domestic Birds: Migrating birds may interact with domestic poultry, leading to the potential transmission of the virus to domestic flocks.
4.5. Cross-Species Transmission: Interactions with diverse bird species enable the virus to adapt and potentially infect new hosts, including domesticated birds.
Monitoring, surveillance, and biosecurity measures are crucial to managing the spread of bird flu, with a focus on both wild bird populations and domestic poultry farms.
- Clinical signs of bird flu, or avian influenza, can vary in severity and may manifest differently in different bird species. In humans, avian influenza viruses like H5N1 and others that have zoonotic potential can cause severe respiratory illness.
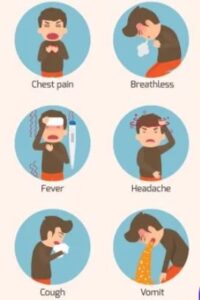
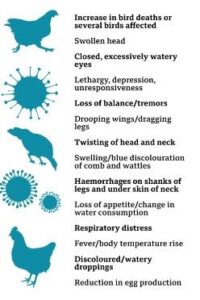
Common clinical signs in birds and, in some cases, humans include:
5.1. Respiratory Symptoms:
-Birds: Respiratory distress, coughing, sneezing, nasal discharge, and difficulty breathing.
-Humans: Severe respiratory symptoms, including cough, shortness of breath, and chest discomfort.
5.2. Decreased Egg Production:-In poultry, a decline in egg production and changes in eggshell quality may be observed.
5.3. Swelling of Head and Neck:
-In birds, particularly waterfowl, noticeable swelling in the head and neck region.
5.4. Edema (Swelling):
-Birds may exhibit edema or swelling in the comb, wattles, and around the eyes.
5.5. Drop in Feed Consumption:
-Reduced appetite and a drop in feed consumption in affected birds.
5.6. Nervous System Signs:
-In severe cases, birds may show neurological signs such as tremors, paralysis, and a twisted neck.
5.7. Lethargy:
-General lethargy and a lack of energy in both birds and humans.
5.8. High Mortality Rates:
-In poultry, avian influenza can lead to a rapid increase in mortality rates, sometimes approaching 100%.
5.9. Zoonotic Transmission:
-In rare cases of zoonotic transmission to humans, symptoms may include high fever, cough, and severe respiratory distress.
- Diagnosis:
Diagnosis of avian influenza is initially based on clinical signs and high flock mortality. Confirmation involves isolating, identifying, and characterizing the virus from suspected samples like tracheal/cloacal swabs, feces, and tissue samples. Definitive diagnosis requires direct detection of avian influenza viral antigen/nucleic acid in affected tissues, swabs, and inoculated cell cultures or embryonating chicken eggs. Techniques such as Hemagglutination (HA) and Hemagglutination Inhibition (HI) tests during virus isolation indicate the virus’s nature and confirm the infection. Various methods like Agar Gel Immunodiffusion (AGID), Immunofluorescence Test (IFT), Immunoperoxidase Test (IPT), and Enzyme-linked Immunosorbent Assays (ELISA) are used to demonstrate viral antigen in clinical samples. Detecting antibodies through AGID and HI tests is valuable. Virus Neutralization Test (VNT), IFT, IPT, and ELISA are important diagnostic tools. Subtyping of avian influenza viruses can be achieved using specific antisera against each of the 17 HA and 10 NA antigens in AGID. Hemagglutination and neuraminidase inhibition (H/NT) tests against a range of antisera are also useful. Molecular tools like Reverse Transcription-polymerase Chain Reaction (RT-PCR), real-time PCR, and PCR-ELISA are employed for subtyping.
7.Treatment: It’s important to note that the treatment of bird flu (avian influenza) in birds primarily involves supportive care, and in humans, it requires prompt medical attention and antiviral medications.
7.1. In birds:
7.1.1. Supportive Care: Providing a stress-free environment, proper nutrition, and maintaining good hygiene.
7.1.2. Culling: In cases of severe outbreaks, culling infected birds may be necessary to prevent the spread of the virus.
7.2. In humans:
7.2.1. Antiviral Medications: Prescription Anti-Flu drugs like Amantadine, Rimantadine, Zanamivir/Relenza and Oseltamivir/Tamiflu) are commonly used to reduce the severity and duration of symptoms. These medications should be administered under medical supervision.
7.2.2. Supportive Care: Rest, hydration, and over-the-counter medications for fever and pain relief may be recommended.
Prevention through vaccination, good hygiene practices, and proper biosecurity measures are key components in managing avian influenza in birds and reducing the risk of zoonotic transmission to humans. In the case of human infection, seeking medical attention promptly and following prescribed antiviral treatments are essential for a better prognosis.
- Prevention and Control of Avian Influenza:
8.1. Key Measures:
-Early disease awareness and detection are essential.
-Culling and stamping out of infected birds, along with proper disposal, are crucial.
-Timely notification, strict biosecurity, isolation, zoning, and quarantine are vital strategies.
-Control of live bird markets and a judicious vaccination approach are recommended.
8.2. Biosecurity Principles:
-Implement isolation, traffic control, and sanitation measures.
-Adhere to cleanliness, good sanitation, and hygienic practices on the farm.
-Ensure suitable decontamination and disinfection procedures.
-Sanitize vehicles before and after arrival to prevent virus spread.
8.3. Preventing Exposure:
-Minimize human traffic and avoid unnecessary visitors.
-Employees should wear clean clothing supplied at the farm daily.
-Disinfectant boot dips should be used to reduce the risk of infection spread.
-Avoid contact of poultry with migratory/wild/free-flying birds and waterfowls.
8.4. Water Management:
-Prevent standing and stagnant water, which attracts migrating waterfowl.
8.5. Education and Surveillance:
-Educate poultry farm employees about the risks of live bird markets.
-Submit sick or dead birds promptly for diagnosis in recognized laboratories.
-Regular surveillance and monitoring of Avian Influenza virus to assess disease status.
8.6. Epidemiological Investigations:
-Conduct epidemiological investigations with strict biosecurity measures to prevent further spread.
8.7. Control Measures:
-Implement stamping out of infected poultry in a designated radius around outbreaks.
-Prohibit sale and transportation of poultry products and close poultry markets in the infected zone.
-Disinfect premises after culling, and restocking should follow specified protocols.
- Bird Flu Outbreak Prevention:
9.1. Immediate Reporting:
-Suspected cases or outbreaks must be promptly reported to regulatory authorities.
-Handling should be left to experienced personnel, including veterinarians and cullers.
9.2. Biosafety Measures:
-Trained professionals wearing protective gear should handle suspected birds.
-Necropsy of affected poultry birds should not be conducted in the field.
9.3. Movement Restrictions:
-Strictly restrict the movement of birds from areas where bird flu is detected.
-Infected or exposed poultry flocks should undergo culling and stamping out.
9.4. Sample Collection and Processing:
-Field veterinarians should be trained for proper sample collection.
-Suspected clinical samples must be processed immediately for timely diagnosis.
9.5. Safety Measures for Handling Dead Poultry:
-Use appropriate safety gear, including gloves, masks, goggles, gowns, and rubber boots.
-Confine live birds being submitted to the laboratory securely.
9.6. Transportation Protocols:
-Dead birds should be securely packed in leak-proof plastic bags and transported under chilled conditions to investigation laboratories.
9.7. Collaboration with Authorities:
-Seek assistance from local animal husbandry authorities for safe burial procedures.
-Monitor and closely observe individuals exposed to bird flu virus and suspicious farms.
9.8. Cross-Border Monitoring:
-Monitor cross-border trade with affected countries and enforce strict regulations or complete bans.
9.9. International Surveillance:
-Implement rigorous surveillance and vigilance for bird flu at international airports, railways, and land transport.
9.10. Public Awareness and Training:
-Mass media involvement for widespread public awareness.
-Organize education and training programs for various stakeholders in the poultry industry.
9.11. Hygiene Measures:
-Emphasize thorough cleanliness and heightened sanitation.
-Encourage regular handwashing with soap/detergent after handling contaminated items.
- Vaccination Strategies:
10.1. Global Use of Inactivated A Vaccines :
-Inactivated A vaccines are widely employed globally for avian species.
-Live conventional influenza vaccines are not recommended for any bird subtype
10.2.Vaccine Types and Development: – -Various inactivated vaccines, including homologous, heterologous, and oil emulsion vaccines, are developed.
-Monovalent and polyvalent vaccines, with adjuvants, effectively induce antibodies.
10.3. Efficacy and Limitations:
-Haemagglutinin (HA)-based vaccines protect against homologous HA subtype viruses.
-Limited efficacy is observed against heterologous HA viruses.
10.4. Use Against High Pathogenic Avian Influenza (HPAI):
-Increasing use of vaccines against HPAI in countries with large outbreaks of Highly Pathogenic Notifiable Influenza (HSNI).
10.5. Routine Application in Poultry Production:
-Routine application of AI vaccines in poultry production systems is uncommon.
10.6. Goals of Commercial AI Vaccines:
-While commercial AI vaccines cannot entirely prevent virus infection, they serve multiple goals when appropriately employed.
10.7. Recent Vaccine Advancements:
-Advancements include recombinant vaccines, DNA vaccines, reverse genetics-based vaccines, vector vaccines, and subunit vaccines.
10.8. Differentiating Infected from Vaccinated Animals (DIVA) Strategy:
-Marker vaccines play a key role in DIVA strategy.
-Incorporation of a distinct Neuraminidase (NA) helps differentiate from field virus infection.
10.9. Gene-Deleted Mutants in Live Bird Flu Vaccines:
-Gene-deleted mutants contribute to live bird flu vaccines but pose risks of gene reassortment with field viruses.
10.10. Vectored Bird Flu Vaccines:
-Vectored vaccines using various viruses expressing the H5, H7 of bird flu virus hemagglutinin gene.
-Replication issues observed in birds with prior exposure.
10.11. Reverse Genetics Techniques:
-Crucial role in developing candidate vaccine viruses against HPAI viruses, including H5N1 and HSNI subtypes.
-Reassortant viruses aid in differentiating infected and vaccinated birds (DIVA strategy).
10.12. Recombinant NDV for Avian Influenza and Newcastle Disease Control:
-Recombinant Newcastle Disease Virus (NDV) expressing HA of AIV/H5N1 can help control avian influenza and Newcastle Disease.
-India focuses on culling and containment rather than widespread vaccination
Conclusion: Bird flu outbreaks pose a global threat, requiring swift and collaborative efforts. Recent H5N1 cases signify increasing dangers, especially in Southeast Asia. Prevention strategies focus on biosecurity, surveillance, and vaccination. Timely, international cooperation is crucial for effective control, utilizing advanced diagnostics and biotechnology. The goal is to safeguard the poultry industry and prevent a potential deadly pandemic.
References:
1) Artois, M., D. Bicout, D. Doctrinal, R. Fouchier and D. Gavier-Widen et al., 2009. Outbreaks of highly pathogenic avian influenza in Europe: the risks associated with wild birds. Rev. Sci. Tech.Off.int.Epiz.,28:69-92. 2) Babiuk, L.A., S. Gomis and R. Hecker, 2003. Molecular approaches to disease control.Poult. Sci,82:870-875.
3) Bano, S., K. Naeem and S.A. Malik, 2003. Evaluation of pathogenic potential of Avian Influenza virus serotype H9N2 in chickens. AvianDis.,47:817-822
4) Beato, M.S.,I.CapuaandI).J.Alexander,2009 Avian influensa viruses in poultry products: A review.AviPathol.,38:193-200.
4) Capua, I., C. Terregino, G. Cattoli, F. Mutinelli and J.F. Rodriguez, 2003a. Development of a DIVA (Differentiating Infected from Vaccinated Animals) strategy using a vaccine containing a heterologous neuraminidase for the control of avian influenza.Avian Pathol., 32: 47-55.
5) Capua, 1. and D.J. Alexander, 2004. Avian influenza:Recent developments. Avian Pathol., 33: 393-404.
6) Capua, I.,G. Cattoli and S. Marangon, 2004. DIVA-a vaccination strategy enabling the detection of field exposure to avian influenza. Dev. Biol. (Basel),119: 229-233.
7) Capua, 1. and D.J. Alexander, 2007. Avian influenza infections in birds-a moving target. InfluenzaResp.Vir.,1:11-18
8) Capua, I. and D.J. Alexander, 2009. Avian influenza infection in birds: A challenge and opportunty lor the poulty veterinarian.Poult.Sei.,88:842-846.
9)Dahlhausen, B., 2010. Future veterinary diagnostics.J. Exotic. Pet. Med., 19: 117-132.
10) Devnani, M., 2012. Factors associated with the willingness of health care personnel to work during an influenza public health emergency: An integrative review. Prehosp. Disaster Med., 27: 551-566.
11) Dhama, K., R.S. Chauhan, J.M. Kataria, M. Mahendran and S.Tomat, 2005. Avian Influenza: The current perspectives. J. Immunol. Immunopathol., 7: 1-33.
12) Dhama, K. and M. Mahendran, 2008. Technologies and advances in diagnosis and control of poultry diseases: Safeguarding poultry health and productivity. Poult. Technol., 2: 13-16
13) Dhiman, N., M.J. Espy, C.L. Trish, P.A. Wright, T.F. Smith
and F Pritt, 2010. Evidence for amino acid changes in a base-pair region of highly conserved matrix gene of pandemic (HIN1) 2009 influenza a virus.J. Cil Microbiol., 48: 3817-3819.
14) Dhama, K., A.K. Verma, S. Rajagunalan, R. Deb and K. Karthik et al., 2012a. Swine flu is back again: A review. Pak. J. Biol. Res., 15: 1001-1009.
15) Dhama, K., R. Tiwari and S.D. Singh, 2012b: Biosecurity measures at poultry farms and thumb rules to avoid developing a serious zoonotic illness from birds.Poult. Punch., 28: 30-51.
16) Dhama, K., A.K. Verma, R. Tiwari, S. Chakraborty and R. Vora et al., 2013a. Applications of Geographical Information System (GIS)-An advanced tracking tool for disease surveillance and monitoring in veterinary epidemiology: A perspective (2013). Adv. Anim. Vet.Sci., Vol. 1, (In Press).
17) Dhama, K., S. Chakraborty, S. Kapoor, R. Tiwari and A.K. Verma et al., 20136. One world one health-veterinary perspectives. Adv. Anim. Vet. Sci.,(In Press)
18) Forrest, H.L. and R.G. Webster, 2012. Perspectives on influenza evolution and the role of research. Anim.Health Res. Rev., 11: 3-18. 19) Fouchier, R.A.M., A. Garcia-Sastre and Y. Kawaoka, 2012.The pause on avian H5N1 influenza virus trasmission research should be ended. M. Biol.,Gamblin, S.J. and J.J. Skehel, 2010. Influenza hemagglumi and neuraminidase membrane glycoprotems. J. Biol. Chem., 285: 28403-28409. 20) Ge, J., G. Deng, 7. Wen. G. Tian and Y. Wang et al., 2007 Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous HSN Avion influenza viruses. J. Virol., 81: 150-158 21) Hsu, J., N. Santesso, R. Mustafa, J. Brozek and Y.L. Chen et al., 2012. Antivirals for treatment of influenza: A systematic review and meta-analysis of observational studies. Ann. Intern. Med., 156: 512-524.
22) Ibrahim, A.K., A.I. Youssef, A.S. Arafa, R. Foad and M.M. Radwan et al., 2012. Anti-H5N1 virus new diglyceride ester from the Red Sea grassThallasodendron ciliatum. Nat. Prod. Res.,
Iwami, S.; Y. Takeuchi and X. Liu, 2008. Avian flu pandemic: Can we prevent it? J. Theor Brol,
257: 181-190
23) Jathult, J.D., 2012. Oseltamivir (Tamiflu(B)) in the environment, resistance development m influenza A. viruses of dabbling ducks and the risk of transmission of an oseltamivir-resistant virustohumans:Areview.infect.Ecol.Epidemiol.,10.3402/ee.v210.18385
24) Kataria, J.M., S.A. Sylvester, K. Dhama, B.B. Dash and S.K. Gupta, 2005b. Bird flu-pathogenicity, epidemiology, diagnosis and control. Proceedings of the Seminar cum Workshop on Recent Advances and Trends in the Field of Animal Disease and Control, February 23-24, 2005, Department of Animal Husbandry, pp: 10-24.
25) Kim, HM., J.H. Oh and S.H. Seo, 2008. Genetic characterization of Avian influenza viruses isolated from waterfowl in southern part of South Korea in 2006.VirusGenes,37:49-51.
26) Kodihalli,S.,D.L.KobasaandR.G.Webster,2000.Strategies for inducing protection against avian influenza A virus subtypes with DNA vaccines. Vaccine,18:2592-2599
27) Lee, C.W., D.A. Senne and D.L. Suarez, 2004. Generation of reassortant influenza vaccines by reverse genetics that allows utilization of a DIVA. (Differentiating Infected from Vaccinated Animals) strategy for the control of avian influenza. Vaccine, 22: 3175-3181.
28) Lee, Y.J., Y.K. Choi, Y.J. Kim, M.S. Song, O.M. Jeong arid E.K. Lee, 2008. Highly pathogenic Avian influenza virus (H5N1) in domestic poultry and relationship with migratory birds, South Korea. Emerg. Infect.Dis., 14: 487-490
29) Liu, J., Y. Bi, K. Qin, G. Fu and J. Yang et al., 2009 Emergence of European Avian influenza virus-like HINI swine influenza a viruses in China. Am. Soc Microbio., 47: 2643-2646
30) Murugkar, H.V., S. Nagarajan, C. Tosh, S. Bhatia and G. Venkatesh et al-2008. HSN1 virus outbreaks in poultry in India. Vet. Rec., Vol. 162, Musa, O.I., A.G. Salaudeen A.A.
Akanbi and O.A. Bolarinwa, 2009. Risk factors, threats and prevention of highly pathogenic avian influenza (hpai) in African countries. Afr. J. Cln Exp.Microbiol., 10: 99-116. 31) Nicolson, C.,D. Major, J.M. Wood and J.S. Robertson,2005. Generation of influenza vaccine viruses on Vero cells by reverse genetics: An HSNI candidate vaccine strain produced under a quality system.Vaccine, 23: 2943-2952
32) OIE, 2012. Update on highly pathogenic avian influenza in animals (type HS and H7). World Orgnization for Animal Health.
33) Perdue, M.L. and D.E. Swayne, 2005. Public health risk from Avian influenza viruses. Avian Dis., 49: 317-327.
34) Postel, A., T. Letzel, S. Frischmann, C. Grund, M. Beer and T. Harder, 2010. Evaluation of two commercial loop-mediated isothermal amplification assays for detection of avian influenza H5 and H7 hemagglutinin genes. J. Vet. Diagn Invest., 22: 61-66.
35) Rashid S., K. Naeem, Z.. Ahmed, N. Saddique, M.A. Abbas and S.A. Malik, 2009. Multiplex polymerase chain reaction for the detection and differentiation of Avian influenza viruses and other poultry respiratory pathogens. Poult. Sci.88: 2526-2531
36) Swayne, D.E., M. Gracia, J.R. Beck, N. Kinney and D.L. Suarez, 2000b. Protection against diverse highly pathogenic HS Avian influenza viruses in chicken immunized with a recombinant fowlpox vaccine containing an H5 avian influenza hemagglutinin gene insert. Vaccine, 18: 1088-1095.
37) Swayne, DE., 2004. Application of new vaccine technologies for the control of transboundary diseases. Dev. Biol., 119: 219-228.
38) Takekawa, J.Y., S.A. Iverson, A.K. Schultz, N.J. Hill, C.J. Cardona, W.M. Boyce and J.P. Dudley, 2010 Field detection of Avian influenza virus in wild birds:Evaluation of a portable iRT-PCR system and freeze-dried reagents. J. Virol. Methods, 166: 92-97.
39) Taubenberger, J.K. and D.M. Morens, 2010. Influenza:The once and future pandemic. Public Health Rep.,125: 16-26.
40) Taubenberger, J.K. and J.C. Kash, 2010. Influenza virus evolution, host adaptation and pandemic formation.Cell. Host Microbe, 7: 140-451.
41) Vandegrift, K.J., S.H. Sokolow, P. Daszak and A.M. Kilpatrick, 2010. Ecology of Avian influenza viruses in a changing world. Arn. N. Y. Acad. Sci.,1195: 113-128.
42) Veits, J., D. Luschow, K. Kinderman, O. Werer, J.P. Teifke, T.C. Mettenleiter and W. Fuchs, 2003.Deletion of the non-essential ULO gene of infectious laryngotracheitis (ILT) virus leads to attenuation in chickens and ULO mutants expressing influenza virus haemagglutinn (H7) protect agaist IL.T and fowl plague. J. Cen. Virol., 84: 3343-3352.
43) Vijayknshna, D., J. Bahl, S. Riley, L. Duan and J.X. Zhang et al., 2008. Evolutionary dynamics and emergence of panzootic HSNI influenza viruses. PLos Pathog, Vol.4 10.1371/journal ppat. 1000161
44) WHO, 2005. Global influenza preparedness plan. The Role of WHO and Recommendations for National Measures before and during Pandemics,WHO/CDS/CSR/GIP/2005.5.
45) WHO, 2008. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO.http://www.who.int/csr/disease/avian_influenza/country/cases
46) WHO,2011.Avian influenza. http://www.who.int/mediacentre/factsheets/avian_influenza/en/
47) WHO/OIE/FAO H5N1 Evolution Working Group, 2012 Continued evolution of highly pathogenic avian influenza A (H5N1): updated nomenclature. Influenza Other Respiratory Viruses, 6: 1-5
48) Weber, T.P. and N.I. Stilianakis,2007. Ecologic immunology of avian influenza (H5N1) in migratory birds. Emerg. Infect. Dis.,13: 1139-1143
49) Webster, R.G., W.J. Bean O.T. Gorman, T.M. Chambers and Y. Kawaoka, 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev., 56: 152-179.
50) WHO/OIEFAO HSN1 Evolution Working Group, 2012 Continued evolution of highly pathogenic avian influenza A (H5N1): updated nomenclature. Influenza Other Respiratory Viruses, 6: 1-5.
51) Weber, T.P. and N.I. Stilianakis, 2007. Ecologic immunology of avian influenza (H5N1) in migratory birds. Emerg. Infect Dis., 13: 1139-1143.
52) Webster, R.G., W.J. Bean, O.T. Gorman, T.M. Chambers and Y. Kawaoka, 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev., 56: 152-179.
53) World Health Organization Global Influenza Program Surveillance Network, 2005. Evolution of HSNI Avian influenza viruses in Asia. Emerg. Infect. Dis.,11: 1515-1521.
54) Yang, P., Y. Duan, P. Zhang, Z. Li and C. Wang et al.,2012. Multiple-CLADE H5N1 influenza split vaccine elicits broad cross protection against lethal influenza virus challenge in mice by intranasal vaccination.PLoS One. Vol. 7 10.1371/joumal. pone.0030252
54) https://www.researchgate.net/figure/Transmission-Cycle-for-Bird-Flu-US-National-Library-of-Medicine-Open-Access-License_fig8_286931468
55) http://rr-middleeast.woah.org/en/our-mission/one-health/highly-pathogenic-avian-influenza/



