Post mortem examination of poultry birds with important gross lesions and sample collection during postmortem for laboratory tests
Kanika Kalai, Assistant Professor, Veterinary Pathology, C.V.Sc & A.H, R. K. Nagar, West Tripura-799008
Email: kanikakalai@gmail.com
Examination of the dead body by systematic dissection of the carcass is called post mortem examination. In veterinary practice post mortem examination of animals and birds is called necropsy. For humans the term “autopsy” is used. The objective of post mortem examination is to ascertain the exact cause of death.
Post mortem examination is necessary to conduct during the following condition:
1. Disease outbreak: It is crucial to conduct a thorough post-mortem examination supported by laboratory tests for an accurate diagnosis during any disease outbreak. Once the disease is properly diagnosed, it can be managed through proper control strategy.
2.Insured animals/birds -It is essential to conduct post-mortem examinations on all insured animals or birds following their death as post mortem report of a dead animal or bird is considered as death certificate.
3.Government farm animals – Post-mortem examination of Govt. animals/birds are necessary to keep the record as a death certificate, which can be used for future reference.
4.Experimental animals – Post-mortem examination of experimental animals/birds is necessary to find out the result of the research.
5.Zoo animals/ wild animals in sanctuaries: Post-mortem examinations of animals or birds in national parks, sanctuaries and zoo are necessary to maintain records akin to death certificates, which can be utilized for future reference.
6.Vetero-legal cases– In the event of a police case p.m. examination of the carcass is necessary. The legal cases may be of two types:
a) Death due to manual injury.
b) Malicious poisoning/ killing.
7.In the slaughterhouse to check the suitability of the meat for consumption.
Post mortem technique is the standard process and system of the post mortem examination of different animals which slightly vary depending on the animal and depending on the purpose and types of postmortems. It is the best, least costly technique to know the health status and control & check any disease outbreak in a poultry farm. History is very important for interpretation of a post mortem examination. So, before starting the examination history, background and any clinical signs of the case should be recorded. Post mortem should start as soon as possible after death to avoid the PM changes in the body which may make the interpretation difficult.
Post mortem technique
For post mortem of birds, the person conducting the PM should be with the personal protective materials such as gloves, apron, mask, caps etc. to protect oneself and the reduce the spread of any infectious disease and contamination of the environment. The materials should be properly disposed off and washed as necessary.
Equipment for postmortem (Fig.1):
- Small and large straight scissors with pointed as well as blunt ends.
- Curved scissors.
- Small and large knives.
- Scalpel or blade.
- Bone cutter and a saw.
- Small and large forceps.
- Tooth forceps.
- Hand lens.
- Hand gloves – rubber or latex.
- Bunsen burner or spirit lamp or stove.
- Syringes and needles.
- Spirit or alcohol.
- Cotton and cotton swabs. Sterile swabs, vials, petri dishes and test tubes. (swab for bacteria and virus are different)
- Pasteur pipettes and rubber bulbs.
- Fixatives like 10% formalin, formol saline or netural formalin.
- Clean glass slides and coverslips.
- Different staining sets and stains like Gram’s, Ziehl – Neelsen, Giemsa and Wright’s stain.
- Normal saline and glycerine saline.
- Small and large trays.
- Matches and torch.
- Disinfectants like dettol, savlon, phenol etc.
- Insecticides or electric flytrap for controlling fly population.

Figure 1. Post mortem equipment/materials
For a proper outcome of the pm examinations there are few principles that need to be followed:
Adequate light: PM should be performed in adequate light preferably day light and if necessary, then only artificial lights should be used.
Observation/visualisation: The carcass and the visceral organs should be examined minutely for any kind of deviation than the normal structure.
Palpitation: Checking the consistency of the organ by palpation is very important e.g. consistency of lungs will change in pneumonia.
Cut surface: Checking of the cut surface by incision is very important to check any oozing or to know the change in the parenchyma of visceral organs.
Smell: Odour is also important to assess the time of death and for some abnormality in the stomach contents.
Histopathology and other laboratory tests: For confirmation of the disease condition and aetiology.
Few more salient points for conducting a PM
- PM should not be conducted inside the farm building to avoid spreading of any infection.
- A designated space should be allocated in organised farms for PM
- It should be an elevated place with proper light.
- Personal protection is necessary.
- Anything from the PM area should not be used in the farm without proper sterilisation.
- Proper biosecurity measures should be followed to prevent any contamination and spread of infectious agents.
- It is preferable that someone second should note the changes and lesions and photograph the organs as the postmortem progresses
Step by step procedure of postmortem:
It is necessary to examine fresh carcasses. In large flock it is better to bring live moribund birds and some birds showing little symptoms and healthy birds to know the exact lesions and aetiology of death. For sacrificing live birds several humane methods are followed like cervical dislocation and euthanasia by inhalation and injection. There are several variations in the pm techniques of birds and animals depending on the situation and the veterinarian. A standard protocol is described below:
- The carcass should be placed in post mortem tray on its back (Fig No 1)
- Examined for any external injury, parasites, deformity, parasites, discharge from natural orifice, combs, wattles, dehydration, emaciation etc.
- The dead bird soaked in water and place on tray or pm table again
- A cut is made through the corner of the beak with the blunt point of scissors inside cut down through the neck, pharynx, oesophagus and crops exposing the mucosa.
- The legs stretched outward the way that the legs lie flat on the table.
- Condition of the bones can be checked at this point by breaking the legs. If it is rubbery then nutritional deficiency is present.
- The skin from the vent to the neck is cut exposing the muscles. Also, from the legs exposing thigh muscle.
- From the keel bone the muscles should be cut from both sides lifting the sternum. Some preferred cutting through the sternum by bone cutters cutting the ribs and exposing the internal organs.
- All the visceral organs are exposed by this cutting.
- Internal organs should be checked properly in situ before removing one by one.
- Samples for microbiological analysis are collected maintaining aseptic procedures at this stage.
- The lungs, liver, heart should be removed carefully by gentle traction and with the help of a sharp scalpel or scissor cutting through wherever necessary.
- The GIT is removed in the same way.
- Kidneys, ovaries, oviducts should be observed and removed carefully.
- The trachea should be cut through till the bifurcation and then bronchi, bronchioles, alveoli, parenchyma as much as possible and check for any type of lesions.
- The heart is examined properly and then an incision is made to check the endocardium.
- Liver palpated and checked for any lesion on the surface and by cutting at several points.
- Spleen checked for any abnormality.
- The proventriculus and gizzard opened to check the presence of haemorrhage and other abnormalities.
- The intestines are observed for presence of parasites, lesions in the mucosa and in serosa.
- The bursa should be observed carefully.
- Impression smears can be collected if any septicaemic condition is suspected before opening of GIT.
- Intestinal scraping and cecal scrapings are collected in slides for checking the Eimeria oocyst under microscope.
- All the organs should be collected for histopathological examination.
- Small pieces of samples with lesions along with surrounding healthy-looking tissue should be collected in 10% buffered formalin for fixation of the tissues.
- The brain can be examined and collected for histopathological examination.
A tentative diagnosis can be made based on the post mortem examination and history of the case. For confirmatory diagnosis it is necessary to establish it through laboratory tests like culture, isolation, serology, molecular diagnosis.
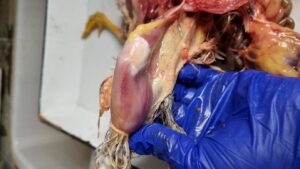
Fig 2. Haemorrhage in muscle (thigh and breast muscle)
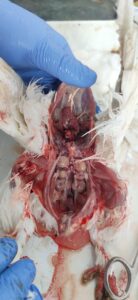
Figure 3. Swollen kidneys with necrotic spots
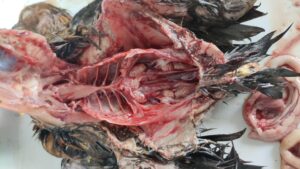
Figure 4. Swollen and pale kidneys
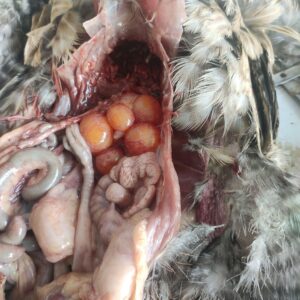
Figure 5. Omphalitis
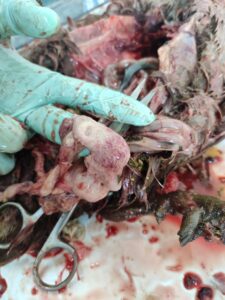
Figure 6. Haemorrhagic salpingitis
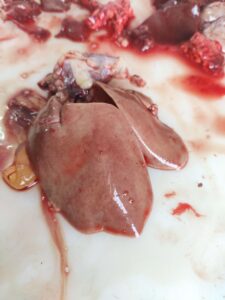
Figure 7. Pale liver with petechial haemorrhage

Figure 8. Opening of brain
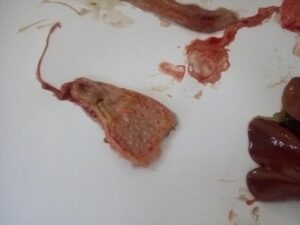
Figure 9. Haemorrhage in the tip of proventricular glands (RD)
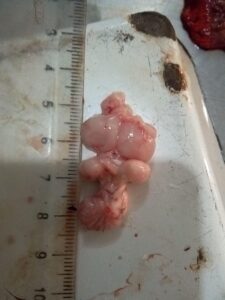
Figure 10. Brain (normal)
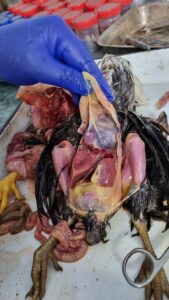
Figure 11. Fibrinous pericarditis, perihepatitis and peritonitis (E coli)
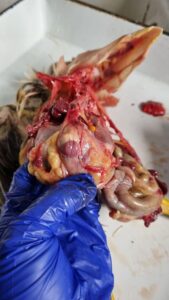
Figure 12. Inflamed and haemorrhagic Bursa of Fabricius (IBD)
Important diseases and their lesions noted during postmortem (Table 1):
| Name of the disease | Aetiological agent | Common gross lesions (alteration in the tissue structure visible during PM) | Materials to be collected for laboratory test | ||||
| Ranikhet | Paramyxo virus type 1 serotype | Pinpoint (petechial) haemorrhage on the tip of proventricular gland, haemorrhage in the cecal tonsil, haemorrhagic enteritis, white necrotic patches in spleen | – Serum for serological test.
– Intestine, trachea, proventriculus, brain for isolation and identification – Pieces of all internal organs for histopathological examination |
||||
| Infectious Bursal Disease of Gumboro disease | Birna virus | Swollen, hemorrhagic bursa, haemorrhage in the thigh and breast muscles, presence of cheesy mass in bursa | Sera and bursa for serological test
Bursa and spleen for vial isolation and identification Bursa, kidneys and other organs for histopathology |
||||
| Infectious bronchitis | Corona virus | Caseous plug in the lower trachea, bronchi or air sac. | Serum
Trachea, lungs, oviduct for isolation Kidney form: swollen, pale kidneys with distended ureter and white urate deposition.
|
||||
| Infectious LaryngoTracheitis | Herpes virus | Tracheitis with a haemorrhage and congestion. It may contain cheesy exudate. | Serum
Traheal exudate Tachea |
||||
| Fowl pox | Avipox virus | Skin form: pock lesions in combs and wattles | Sera
Pock nodule along with pieces of skin and other organs Tissues with the lesion |
||||
| Inclusion body hepatitis | Group l Adenovirus | Friable, pale and enlarged liver. Haemorrhage may be present in the liver and muscles. | Sera
Liver, faeces Liver for histopathology |
||||
| Avian Influenza | Avian influenza virus | Marked bluish discolouration of the external organs. Severe congestion in internal organs with haemorrhage in almost all organs including muscles, proventriculus etc. | Sera
Swabs, Intestinal contents PIeces of all internal organs |
||||
| Name of the disease | Aetiological agent | PM lesion | Materials to be collected for laboratory examination | ||||
| Colibacillosis | Escherichia coli | Fibrinous perihepatitis, pericarditis, air sacculitis | Smear examination
Heart, liver and lungs for bacterial isolation and identification |
||||
| Infectious coryza | Haemophilus paragallinarum | Swelling of face. Sinusitis with fibrinopurulent or mucous exudate. | Swabs from infra orbital sinus, trachea, airsac | ||||
| Necrotic enteritis | Clostridium perfringens type A & C | Small intestine greatly thickened with inflammatory exudate and necrosed. | Intestinal scrapping, wall, mesenteric lymph nodes | ||||
| Gangrenous dermatitis | Clostridium septicum and Clostridium perfringens type A | Affected area is dark and moist. Underlying muscle is lysed, discoloured and oedematous. | Exudate on skin and sub cutaneous tissue with underlying muscles | ||||
| Pullorum disease | Salmonella enterica pullorum | Presence of unabsorbed yolk sacs and classic gray nodules in the liver, spleen, lungs, heart, gizzard, and intestine. Firm, cheesy material in the ceca (caecal cores) and raised plaques in the mucosa of the lower intestine are sometimes seen
Adults: irregular, cystic, deformed, pedunculated, discoloured ovary. |
Liver, spleen, ceca
Heart blood |
||||
| Fowl typhoid | Salmonella gallinarum | Liver swollen, friable. A metallic sheen appeared on the surface of the liver.
In chicks inflammation of cecum, spleen, lungs, peritoneum observed. In adult oophoritis, salpingitis, orchitis, peritonitis observed. |
Heart blood.
Liver, spleen, ceca and any organ with lesion |
||||
| Fowl cholera | Pasteurella multocida | Pin point necrotic foci in liver. Liver swollen with haemorrhage | Impression smear from lungs, liver, heart blood
Liver, heart blood |
||||
| Aspergillosis/brooder pneumonia | Aspergillus fumigatus | Fungal nodule in nasal passage, trachea and air sacs. Nodules also appeared in lungs. Dirty greying yellow cheese like exudate may be found in lung nodules. | Granulomatous nodules from the organs
Smear of affected tissue from lesion
|
||||
| Thrush | Candida albicans | Inflammation of crop with presence of white cheesy material | Pieces of crop and esophagus | ||||
| Coccidia | Eimeria sp | Cecal coccidiosis: Ceca is filled with red coloured dirty exudate
Intestinal coccidiosis: Ballooning of the affected part of the intestine with bloody exudate |
Wet examination of intestinal scrapping | ||||
| Gout | Metabolic disturbance | Visceral gout: Urate disposition in visceral organs as chalky gritty material.
Articular gout: Deposition of urate in joints. |
-Tissue for histopathology | ||||
| Ascites | Pulmonary hypertension | Presence of large amounts of fluid in the abdominal cavity. Hydropericardium and enlargement of heart. Lungs and liver are also swollen and congested. | -Tissue for histopathology | ||||
| Heat stress | High environmental temperature, overcrowding | Cooked meat appearance of breast muscle. | Tissue for histopathology | ||||
| Bumblefoot | Staphylococcus aureus | Abscess in foot pad | Lesion | ||||
- For viral culture and isolation materials should be collected in phosphate-buffered isotonic saline containing antibiotic and 50% glycerine and saline.
- For bacterial culture and identification, the samples should be collected in saline solution or directly in nutrient broth. The samples should be collected and transferred in ice.
- For histopathology 10% buffered formalin solution should be used.
Reference:
- Ganti A Sastry (2019). Veterinary Pathology. 7th CBS Publishers & Distributors. India
- Nawab A, Nawab Y, Tang S, Wu J, Liu W, Li G, Xiao M, An L (2018). A pictorial guidebook on poultry diseases; diagnostic techniques and their effective treatment Animal Review, 5(2): 34-5
- Baruah GK and Pathak D C (2011). Postmortem and its importance in disease diagnosis. Compendium of State Level Training Program for Veterinarians under ASCAD on “Control and eradication of some economically important infectious diseases of livestock and poultry”. Khanapara, Ghy.
Vegad J L (2018). Poultry diseases: A guide for farmers & poultry professionals. 2nd edition. CBS Publishers & Distributors. India


