Fluid Therapy in Animals
Dr. Ramesh B. Jagtap1, Dr. D J Kalita2 Dr. Sujit Kumar3
Corresponding Author: 1. Dr. Ramesh B. Jagtap, M.V.Sc. Ph.D.,
Designation: Manager- Technical & Regulatory; Zydus Animal Health and Investments Ltd. (A wholly owned subsidiary of Cadila Healthcare Ltd.)
Email: ramesh.jagtap@zyduscadila.com
2. Dr. D J Kalita, M.V.Sc.
Designation: Head- Technical & Regulatory; Zydus Animal Health and Investments Ltd. (A wholly owned subsidiary of Cadila Healthcare Ltd.)
3. Dr. Sujit Kumar, M.V.Sc.
Designation: Head- Product Promotion- Livestock; Zydus Animal Health and Investments Ltd. (A wholly owned subsidiary of Cadila Healthcare Ltd.)
Introduction
In most of the emergency, critical ill animals, which need intensive care, body fluids containing body water, electrolytes, and acid-base balance are important considerations in the evaluation and treatment in any disease condition. The goals of fluid therapy should be to maintain cardiac output and tissue perfusion, and to correct acid-base and electrolyte abnormalities.
Fluid therapy, particularly Intravenous (IV) fluid administration is probably one of the most frequently used therapies. IV fluid administration have long been considered as fundamental in the management of critically ill and hospitalized animals. However, still research efforts continue to prove whether the type and volume of fluids infused can contribute to morbidities and could decrease the chances of a favourable outcome in critically ill animals especially companion animals.
For decades, crystalloid solutions are the most frequently used by practising Veterinarians, with normal saline (NS) and Ringer’s lactate (RL) being most preferred. Colloids are considered as an alternative to crystalloids, but its use varies depending on number of clinical variables. Nevertheless, Colloids are increasingly become considered indispensable in the management of critically ill patients. Typical indications for colloid administration include animals with tissue oedema, hypovolemia, and low oncotic pressure.
Clinically available colloids have generally exhibited similar effectiveness with crystalloids in maintaining colloid oncotic pressure. However, considering the need of patient, clinicians should note the differences among various available colloid preparations; including physical properties, pharmacokinetics, pharmacodynamics and safety profile of various colloids.
Fluid therapy:
Fluid and electrolyte replacement therapy in livestock is needful during negative fluid balance that is when fluid intake is less than the fluid loss from the body and when fluid intake is not enough to meet the metabolic requirements. Fluid therapy in farm and companion animals is important during perioperative period and other disease conditions causing fluid loss.
Rehydration, replacement of lost electrolytes, and restoration of acid‐base balance are the goals for fluid therapy. Before treating animal, veterinarians should develop the plan for fluid and electrolyte replacement therapy by considering and assessing following important points.
1. Volume and rate of fluid administration:
The optimum fluid volume and rate to be administered can be determined by considering replacement of hydration deficit, calculation of maintenance fluid needs, replacement of ongoing losses and also determination of plasma protein concentration.
The volume required for hydration replacement can be calculated as below.
Fluid requirement in litres to replace hydration deficit = (estimated % dehydration) X (body weight in kg)
Generally, hydration replacement must be done slowly over the period of about 4 hours. Following it, maintenance and ongoing losses administered over the remaining hours in the day. However, in case of hypoproteinaemia and particularly in camels, the hydration replacement fluid administration even need to be more slow, with half of the volume replaced in 4 to 6 hours and the remaining should be given over 12 to 24 hours.
Maintenance of fluid requirements account for daily normal water losses pertaining to urination, defecation, respiration, sweat, and other evaporation losses. These losses differ depending on age and physiologic stage of the animal (lactation, pregnancy). Neonates and young animals have higher total body water compare to adults, and thus need higher volume of maintenance fluid (Gottardo, 2002). Generally. maintenance fluid needs can be estimated as below (Jones and Navarre, 2014):
Adults: 50 mL/kg/24 hours or 1 mL/lb/h
Neonates: 70 to 80 mL/kg/24 h or 2 mL/lb/h
These numbers can be followed as guide in all the farm animal species (NRC, 2007). However, in situations where fluid rates are difficult to control or disruption of fluid line, replacement fluids may be divided and administered as single bolus every 3 hours by calculating volume required for 3 hour period. But it may not work in hyper oncotic solutions or parenteral nutrition solutions.
Ongoing losses include loss of fluid, protein, and electrolytes, due to continuing disease process, such as internal haemorrhages, diarrhoea or internal or external loss of fluid, etc., it is difficult to quantify these losses if not able to measure directly. Thus ongoing losses are usually determined indirectly by counting appropriate parameters, like PCV, TPP (total plasma protein), serum electrolyte panel, and body weight may be used to monitor the success of fluid therapy to sustain body fluid, protein, and electrolyte balance.
2. Method or route of fluid administration:
Although in farm animals and camels, large volumes of fluids may be administered orally into the rumen enabling effective treatment of mild to moderate dehydration, parenteral administration is more preferred. Particularly in camels, repeated oral fluids may be stressful and may induce cortisol-mediated lipolysis. In general, oral fluid therapy is beneficial to mentally alert, animals with good gastrointestinal motility, and are less than 8% dehydrated. Other cases of animals are best managed with at least initial parenteral fluid resuscitation and correction of acid-base and electrolyte abnormalities.
3. Type of fluid:
There are 4 major types of fluids used in clinical medicine, which differ in composition, cost and indications. Apart from two well-known fluids that is blood products and parenteral nutrition; two other fluid types required are Crystalloids and Colloids. Considering their importance in critical and emergency medicine, one or multiple type of fluids are required in many illnesses or disease conditions of animals such as anaemia, shock, hypoalbuminemia, hypoglobulinemia, metabolic acidosis, metabolic alkalosis, hyponatremia, hypochloraemia, hypokalaemia, ketosis, hypoglycaemia.
Crystalloid solutions:
In veterinary practice, crystalloid solutions are most commonly used. Generally, crystalloid solutions contain water, electrolytes (in particular Na, Cl), and/or dextrose. Crystalloid solutions may be balanced, resembling composition of extracellular fluid (ECF) for example polyionic solutions like Ringer’s solutions, Normosol, Plasma-Lyte, etc., or unbalanced, meant to substitute specific components, for example isotonic saline (0.9% NaCl), 5% Dextrose solution, etc. Further, crystalloid solutions can be isotonic, for example, normal saline (0.9% NaCl); hypertonic which has higher osmolality than plasma, for example 7.2 % NaCl; acidifying solutions which are used in metabolic alkalosis, for example saline or lactated ringer’s solution; alkalinizing solutions, for example 1.3% or 5% NaHCO3; Dextrose solutions for treatment of hypoglycaemia, for example 5% dextrose.
Colloidal solutions:
A substance with high molecular weight (MW) which mostly retain in the intravascular compartment and thus generates an oncotic pressure is called as colloid. It means oncotic pressure is osmotic pressure generated by high molecular weight compounds in the blood, mostly plasma albumin. These molecules act similar to albumin by maintaining osmotic pressure within the vascular system.
In a healthy animal, colloids persists for longer time in intravascular space compared to crystalloids. But when integrity of capillary membranes altered in certain diseases, colloids may leak out of the vessels.
Generally, colloids are classified into two types; Natural (Plasma albumin) and Synthetic (e.g. gelatin and dextran solutions, hydroxyethyl starch). These colloids possess various characteristics which determine their behaviour in the intravascular compartment.
Despite their higher cost, colloids are more appropriate than crystalloids for use in hypoproteinaemia patients and those requiring longer-term stabilization of cardiac output. In one study of induced haemorrhage and resuscitation in sheep (Smith et al., 1985), the addition of 6% dextran 70 to 7.2% NaCl resulted in maintenance of a significantly higher cardiac output in comparison with other hypertonic solutions by redistribution of interstitial fluid into the vasculature. Administration of 50 mL/kg hetastarch in over 60 minutes to healthy llamas (a camelid) resulted in significant haemodilution, indicating the ability of hetastarch to expand plasma volume (Carney, et al., 2014). These effects were greater than those observed after administration of lactated Ringer’s solution. Hetastarch also significantly increased plasma colloid osmotic pressure for 96 hours after infusion.
• Albumin:
Albumin is a major natural colloid (MW 69 kDa) contributing mainly to normal oncotic pressure in healthy animal. 5% solution is iso-oncotic and leads to 80% plasma expanding effect initially, which persists for 16-24 hours (Martino P., 2007). Being natural colloid, it is associated with lesser side-effects like pruritus, anaphylactic reactions and coagulation abnormalities compared to synthetic colloids. However, cost-effectiveness and in case of septic shock due to increased vascular leakiness, albumin therapy could aggravate and lead to interstitial oedema (Park G, 1996).
• Dextran:
Dextrans are synthetic colloids and are highly branched polysaccharide molecules, available in 6% solution (MW 70 kDa) and 10% solution (MW 40 kDa). Both solutions exert short duration volume expansion effect for 6-12 hrs (Martino, P., 2007) and increase microcirculation by increasing haemodilution and inhibiting erythrocyte aggregation. However, its use is very limited due to potential side effects like anaphylactic reactions, coagulation abnormalities, interfere in blood matching, increase of erythrocyte sedimentation rate and even acute renal failure is implicated following its use in kidney damage patients (Barron and Navickis, 2004; Linder P and B. Ickx , 2006).
• Gelatin:
Gelatin is high molecular weight protein formed from hydrolysis of collagen. Although earlier colliods had potential side effects of high viscosity and tendency to gel at low temperatures, modified gelatins like succinylated, urea-cross linked and oxypolygelatins are currently in use. Most of these products are low molecular weight, short-lived and effective for small duration compared to albumin, starch formulations. Apart from this, higher incidence of anaphylactoid reactions, unclear effect on coagulation and circulatory disturbances could limit their uses (Barron and Navickis, 2004; Tabuchi et al., 1995; Gines et al., 1996).
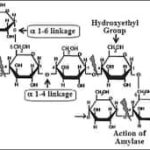
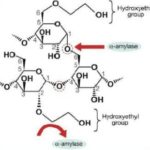
• Hydroxyethyl starches (HES):
HES is derived from amylopectin, which is branched compound of starch. Normally, amylopectin is short-lived (half-life about 20 min) due to rapid hydrolysis by alpha amylase. To increase its persistence in blood and to make the amylopectin molecule more stable, anhydroxyethyl glucose residues are substituted with hydroxyethyl groups mainly at positions C2 and C6. Nowadays safer and more effective HES products are developed, for example 10% HES 200/0.5 or 6% HES 130/0.4. These parameters are highly relevant respect to pharmacokinetics and pharmacodynamics of HES. 6% HES is iso-oncotic with 1:1 volume effect with infused volume and 10% is hyper-oncotic. Like other available synthetic colloids, HES are polydisperse, containing particles with a wide range of molecular mass. Mean MW of the available products ranges from over 670 kDa to 70 kDa.
Molar substitution ratio ranges low 0.4 or high upto 0.7 in available HES preparations. It refers to the modification of the original amylopectin polymer by the addition of hydroxyethyl groups on its glucose units. HES have a varying number of hydroxyethyl residues attached to the anhydrous glucose particles within the polymer. MS is nothing but the average number of hydroxyethyl residues per glucose subunit. The number 0.6 in HES denotes there are seven hydroxyethyl residues substituted on per average 10 glucose subunits. So, such HES preparations referred as Hexastarches, likewise pentastarch (MS 0.5) and tetrastarch (MS 0.4). As more molar substitution, greater the resistance to degradation by alpha-amylase, and consequently, prolongs the intravascular retention time. Therefore, older generation HES products with high MS accumulate in the plasma, unlike the latest generation of tetrastarches and thus lead to potential side effects.
Other important parameter is C2/C6 ratio, refers to carbon atom in glucose residue where substitution of hydroxyethyl has occurred. The higher the C2/C6 ratio, it will create more hindrance to cleavage by alpha amylase, lead to increase in half-life and thus longer persistence in the blood (Fig.1).
HES administration results in increase in oncotic pressure and similar volume expansion compare to 5% albumin preparation and it is greater than that of gelatin. The duration of volume expansion ranges from 8-12 hours. Compare to other colloids like albumin it is cost-effective. Maximum volume which can be transfused of medium weight or so called third generation HES (130 kDa) with medium degree of substitution (0.4) is 50 ml/kg. This is greater as compared to other synthetic colloids like dextrans.
(Fig.1 (a) Structure of HES; (b) Importance of C2:C6 ratio in HES metabolism: The substitution of hydroxyethyl molecules on C2 of glucose residue resist metabolic cleavage by alpha amylase.)
There are several reports which highlight the safety concerns and side- effects of HES formulations in humans and pet animals like coagulopathies, accumulation in different tissues, anaphylactoid reactions, renal impairment (Barron and Navickis, 2004; Treib etal. 1997; Sirti et al., 1999; Schortgen F, et al., 2001; Davidson IJ., 2006). However, there are not many studies or reports proving these side effects in farm animals including equines. In equines, HES potentially increase and maintain colloid osmotic pressure and thus enable its use in conditions like hypoalbuminemia, perioperative fluid replacement or even for fluid resuscitation in haemorrhagic shock (Galen and Hallowell, 2017). Although, HES benefit is recognised in the particular equine cases, however, considering species differences and limited species-specific clinical studies, it should be used judiciously. Further, it is important to consider the data for individual products and not to extrapolate reports from one HES type to another. Clinical studies have revealed significant differences between the HES generations regarding coagulation, tissue storage, and renal function. The third generation HES products clearly stand out with negligible side effects and proving safe (Westphal et al., 2009; James MFM 2008) if used as per recommendations.
Summary and conclusion:
Fluid dynamics, acid-base status and electrolyte balance are similar in all domestic large animals. As fluid is lost largely from the extracellular space, dehydration occurs and the loss of extracellular fluid (plasma) results in hypovolemia and poor cellular perfusion. During fluid loss, most veterinary practitioners’ use crystalloid solutions which may be balanced, unbalanced solutions, hypertonic, isotonic solutions, etc., depending on need of therapy. However, most crystalloids are not confined to the intravascular space and can readily pass through. On the other hand, colloid solutions are excellent for sustained expansion of plasma volume, which is in marked contrast to the use of crystalloid solutions.
The introduction of synthetic colloid solutions for intravenous use as plasma volume expanders has been recognized as one of the great medical advances in clinical medicine. Colloid solutions are used to maintain and or to restore blood volume. Further, in certain cases colloid solutions can be advantageous over blood transfusion owing to the absence of immunologic reactions, longer shelf-life, cost-effectiveness, and reduced risk of infection. Like human, animals do require plasma volume expanders in case of emergency of hypovolemia that may arise during severe bleeding, hypoproteinaemia, shock, traumatic injury, ascites, septic conditions etc. For management of such emergency, in perioperative or critically ill animals, colloidal solution like Hydroxyethyl starch (HES), especially medium molecular weight tetrastarch (130 kDa) is widely used for expansion of intravenous perfusion pressure and thus to avoid vascular collapse enabling oxygen delivery on emergency basis. Conventional crystalloid solutions like Ringer’s lactate or NSS alone are not appropriate to respond in such situation.
References:
Barron ME, Wilkes, Navickis RJ., 2004. A systematic review of the comparative safety of colloids. Arch Surg; 139:552–563.
Carney KR, McKenzie EC, Mosley CA, et al. 2011. Evaluation of the effect of hetastarch and lactated Ringer’s solution on plasma colloid osmotic pressure in healthy Llamas. J Am Vet Med Assoc; 238:768–72.
Davidson IJ., 2006; Renal impact of fluid management with colloids: a comparative review. Eur J Anaesthesiol; 23:721–738.
Galen G.B. and Hallowel, G., 2019, Hydroxyethyl starches in equine medicine, J Vet Emerg Crit Care. 2019; 29:349–359.
Gines A, Fernandez-Esparrach G, Monescillo A, et al., 1996. Randomized trial comparing albumin, dextran-70 and polygeline in cirrhotic patients with ascitis treated by paracentesis. Gasteroenterology; 111:1002–10.
Gottardo F, Mattiello S, Cozzi G, et al. The provision of drinking water to veal calves for welfare purposes. J Anim Sci 2002;80(9):2362–72.
James MFM. 2008.The role of tetrastarches for volume replacement in the perioperative setting. Curr Opin Anaesthesiol; 21:674–678.
Jones, M., and Christine Navarre, 2014. Fluid Therapy in Small Ruminants and Camelids, Vet Clin Food Anim., 30 (2014) 441–453.
Linder P and B. Ickx, 2006. The effects of colloid solutions on hemostasis. Can J Anesth.,53:30–s39.
Martino P, editor. The ICU Book. 3rd edition. Philadelphia: Churchill Livingstone; 2007. Colloid and crystalloid resuscitation; pp. 233–54.
Nutrient requirements of small ruminants: sheep, goats, cervids, and New World camelids. Washington, DC: The National Academies Press; 2007.
Park G. 1996, Molecular mechanisms of drug metabolism in the critically ill. Br J Anaesth; 77:32–49.
Schortgen F, et al. 2001; Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomized study. Lancet, 357:911–16.
Sirti C, Laubenthal H, Zumtobel V, Kraft D, Jurecka W., 1999. Tissue deposits of hydroxyethyl starch (HES): dose- dependent and time-related. Br J Anaesth ;82:510–513.
Smith J, Kramer GC, Perron P, et al., 1985. A comparison of several hypertonic solutions
for resuscitation in bled sheep. J Surg Res., 39:517–28.
Tabuchi N, Haan J, Gallandat RC, Boonstra PW, van Oeveren W.,1995. Gelatin use impairs platelet adhesion during cardiac surgery. Thromb Haemost, 74:1447–51.
Treib J, Haass A, Pindur G. Coagulation disorders caused by hydroxyethyl starch. Thromb Haemost. 1997;78:974–83.
Westphal M, James MFM, Kozek-Langenecker SA, Stocker R, Guidet B, Van Aken H. 2009, Hydroxyethyl starches. Different products-different effects. Anesthesiology;111:187–202.